
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Ta có : \(M_{kk}=\dfrac{20.32+80.28}{20+80}=28,8\) ( đvc )
\(\Rightarrow d_{\dfrac{KK}{H2}}=\dfrac{M_{kk}}{M_{h2}}=\dfrac{28,8}{2}=14,4\)
Vậy ...

a) Ta có: \(M_{XCO_3}=4\cdot25=100\) \(\Rightarrow M_X=100-12-16\cdot3=40\left(đvC\right)\)
\(\Rightarrow\) X là Canxi (Ca)
b) \(\%O=\dfrac{16\cdot3}{100}\cdot100\%=48\%\)
Bài 3:
a) M(XCO3)=25. M(He)= 25.4=100(đ.v.C)
Mặt khác: M(XCO3)=M(X)+ 60
=> M(X)+60=100
<=>M(X)=40(đ.v.C)
=> X là Canxi (Ca=40)
b) %mO=[(3.16)/100].100=48%

PTK(hợp chất)= 3:17,647%= 17(đ.v.C)
Mặt khác: PTK(hc)=NTK(X)+3
<=>17=NTK(X)+3
<=>NTK(X)=14(đ.v.C)
Vậy X là nito (N)
b) PTK(hc)=17(đ.v.C)
Anh Đạt đẹp trai chúc em học tốt!

a)
$n_{Nito} = \dfrac{6,02.10^{23}}{6,02.10^{23}} = 1(mol)$
$m_{Nito} = 1.14 = 14(gam)$
b)
$n_{Cl} = \dfrac{6,02.10^{23}}{6,02.10^{23}} = 1(mol)$
$m_{Cl} = 1.35,5 = 35,5(gam)$
c)
$n_{H_2O} = \dfrac{6,02.10^{23}}{6,02.10^{23}} = 1(mol)$
$m_{H_2O} = 1.18 = 18(gam)$
a) \(n_{N_2}=\dfrac{6,02.10^{23}}{6,02.10^{23}}=1\left(mol\right)\)
=> \(m_{N_2}=1.28=28\left(g\right)\)
b) \(n_{Cl_2}=\dfrac{6,02.10^{23}}{6,02.10^{23}}=1\left(mol\right)\)
=> \(m_{Cl_2}=1.35,5.2=71\left(g\right)\)
c) \(n_{H_2O}=\dfrac{6,02.10^{23}}{6,02.10^{23}}=1\left(mol\right)\)
=> \(m_{H_2O}=1.18=18\left(g\right)\)
d) \(n_{CaCO_3}=\dfrac{6,02.10^{23}}{6,02.10^{23}}=1\left(mol\right)\)
=> \(m_{CaCO_3}=1.100=100\left(g\right)\)


10
\(n_{Zn}=\dfrac{3,25}{65}=0,05\left(mol\right)\)
\(n_{CuO}=\dfrac{6}{80}=0,075\left(mol\right)\)
\(pthh:Zn+HCl->ZnCl_2+H_2\)
0,05 0,05
\(pthh:CuO+H_2\underrightarrow{t^o}H_2O+Cu\)
LTL : \(\dfrac{0,075}{1}>\dfrac{0,05}{1}\)
=>> CuO dư
theo pthh : \(n_{Cu}=n_{H_2}=0,05\)(mol)
=> \(m_{Cu}=0,05.64=3,2\left(g\right)\)
=> \(m_{CuO\left(d\right)}=\left(0,075-0,05\right).80=2\left(g\right)\)
Câu 10:
\(a) n_{Zn} = \dfrac{3,25}{65} = 0,05 (mol)\\n_{CuO} = \dfrac{6}{80} = 0,075 (mol)\)
PTHH:
Zn + 2HCl ---> ZnCl2 + H2
0,05------------------------->0,05
CuO + H2 --to--> Cu + H2O
LTL: \(0,075>0,05\rightarrow\) CuO dư
b, Theo pthh: \(n_{CuO\left(pư\right)}=n_{Cu}=n_{H_2}=0,05\left(mol\right)\)
\(\rightarrow m_{Cu}=0,05.64=3,2\left(g\right)\)
\(c) \text{chất dư là CuO}\\ \rightarrow m_{CuO (dư)} = (0,075 - 0,05) . 80 = 2 (g)\)

Đặt \(n_{Al}=x\left(mol\right);n_{Mg}=y\left(mol\right)\)
\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
\(PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\\ Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
Theo đề ta có: \(\left\{{}\begin{matrix}27x+24y=7,8\\\dfrac{3}{2}x+y=0,4\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%_{Al}=\dfrac{0,2\cdot27}{7,8}\cdot100\%\approx69,23\%\\\%_{Mg}=\dfrac{0,1\cdot24}{7,8}\cdot100\%\approx30,77\%\end{matrix}\right.\)

\(n_{KClO_3}=\dfrac{5,5125}{122,5}=0,045\left(mol\right)\\ 2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\uparrow\\ n_{O_2}=\dfrac{3}{2}.0,045=0,0675\left(mol\right)\\ 2Cu+O_2\rightarrow\left(t^o\right)2CuO\\ n_{CuO}=2.0,0675=0,135\left(mol\right)\\ m_{r\text{ắn}}=m_{CuO}=0,135.80=10,8\left(g\right)\)



 Giải giúp mình câu 10 ik giải chi tiết nha
Giải giúp mình câu 10 ik giải chi tiết nha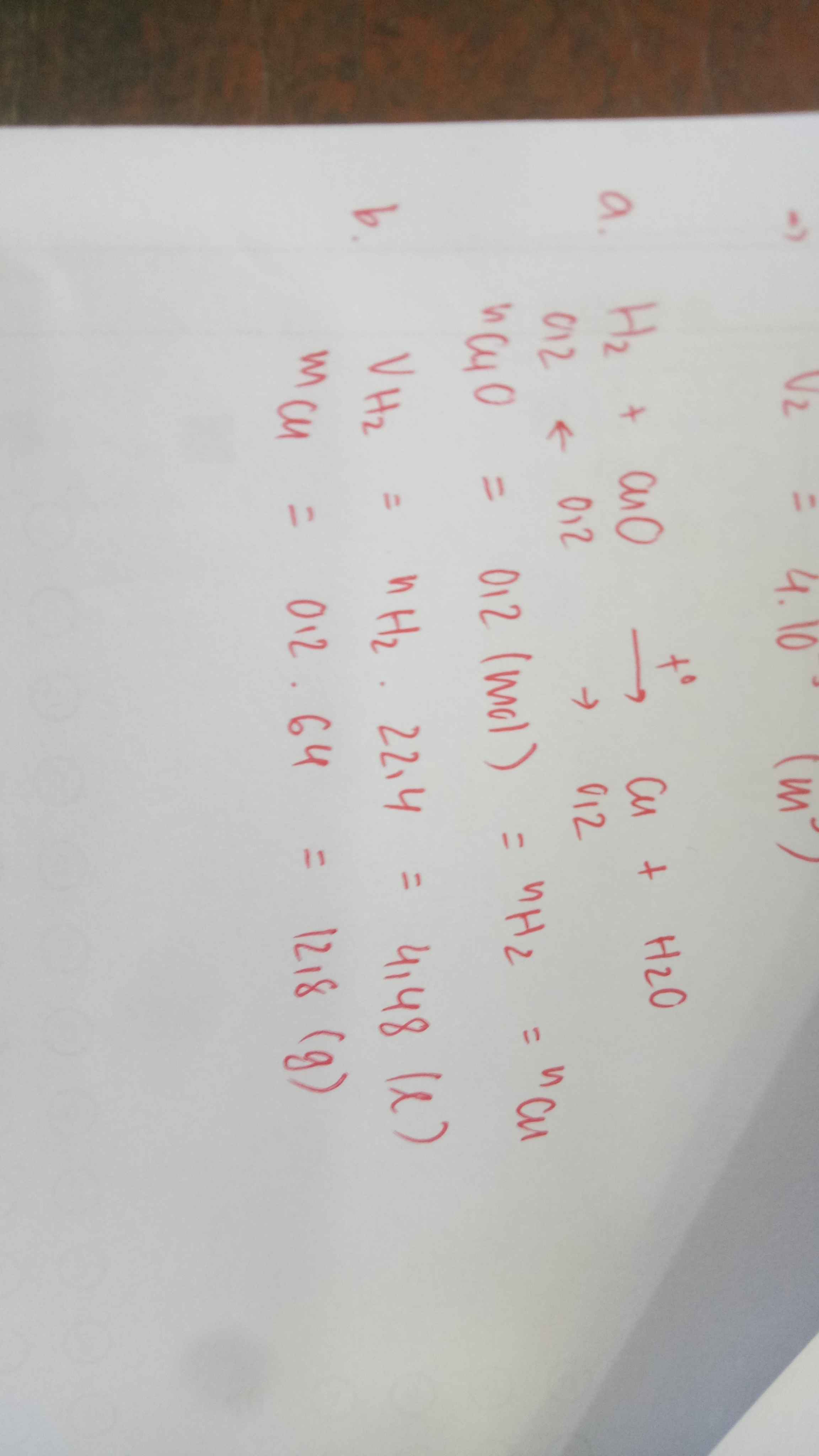
 cau 10 giải chi tiết giúp mình nha
cau 10 giải chi tiết giúp mình nha