Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a. PTHH: \(C+O_2\rightarrow CO_2\)
\(C+\frac{1}{2}O_2\rightarrow CO\)
b. Có \(\hept{\begin{cases}\overline{M}=18,8.2=37,6\\N_{\text{khí}}=\frac{1,12}{22,4}=0,05mol\end{cases}}\)
Theo sơ đồ chiếu \(\frac{n_{CO_2}}{n_{CO}}=\frac{37,6-28}{44-37,6}=\frac{3}{2}\)
\(\rightarrow\hept{\begin{cases}n_{CO_2}=0,03mol\\n_{CO}=0,02mol\end{cases}}\)
\(\rightarrow\hept{\begin{cases}V_{CO_2}=0,03.22,4=0,672l\\V_{CO}=0,448l\end{cases}}\)
c. Theo phương trình \(n_C=n_{CO_2}+n_{CO}=0,05mol\)
\(\rightarrow m_C=12.0,05=0,6g\)

a)
2CO + O2 --to--> 2CO2
2H2 + O2 --to--> 2H2O
b) \(n_{H_2O}=\dfrac{12,6}{18}=0,7\left(mol\right)\); \(n_{CO_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH: 2CO + O2 --to--> 2CO2
0,6<--0,3<------0,6
2H2 + O2 --to--> 2H2O
0,7<--0,35<------0,7
=> \(\left\{{}\begin{matrix}V_{CO}=0,6.22,4=13,44\left(l\right)\\V_{H_2}=0,7.22,4=15,68\left(l\right)\end{matrix}\right.\)
VO2 = (0,3 + 0,35).22,4 = 14,56 (l)
c) \(M_A=\dfrac{0,6.28+0,7.2}{0,6+0,7}=14\left(g/mol\right)\)
=> \(d_{A/O_2}=\dfrac{14}{32}=0,4375\)

a) Gọi $n_{CO_2} = a(mol) ; n_{SO_2} = b(mol)$
Ta có :
$a + b = \dfrac{8,96}{22,4} = 0,4(mol)$
$\dfrac{44a + 64b}{a + b} = 27.2$
Suy ra : a = b = 0,2$
$V_{CO_2} = V_{SO_2} = 0,2.22,4 = 4,48(lít)$
b) Theo PTHH : $n_{K_2SO_3} = n_{SO_2} = 0,2(mol)$
$\Rightarrow m_{K_2SO_3} = 0,2.158 = 31,6(gam)$
Gọi $n_{K_2CO_3} = x(mol) ; n_{Na_2CO_3} = y(mol)$
$\Rightarrow 138x + 106y + 31,6 = 56(1)$
$n_{CO_2} = x + y = 0,2(2)$
Từ (1)(2) suy ra : x = y = 0,1
$m_{K_2CO_3} = 0,1.138 = 13,8(gam) ; m_{Na_2CO_3} = 0,1.106 = 10,6(gam)$

a)
dA/O\(_2\) = \(\dfrac{M_A}{32}\) = 1,25 \(\Rightarrow\) MA = 32 . 1,25 = 40
PTPƯ: C + O2 -----> CO2
C + CO2 -----> 2CO
Trường hợp 1 (Oxi dư)
Ta có: MA = \(\dfrac{44x+\left(1-x\right).32}{1}\) = 40 \(\Rightarrow\) x = \(\dfrac{2}{3}\)
Vậy %VCO\(_2\) = \(\dfrac{2}{3}\) . 100 = 66,67%
%VO\(_2\) = 33,33%
Trường hợp 2 (Oxi thiếu)
MA = \(\dfrac{44x+\left(1-x\right).28}{1}\) = 40 \(\Rightarrow\) x = 0,75
Vậy % VCO\(_2\) = \(\dfrac{a}{a+b}\) . 100 = \(\dfrac{3b}{4b}\) . 100 = 75%
%VCO = 25%
b)
CO2 + CA(OH)2 -----> CaOH3 \(\downarrow\) + H2O
0,06 \(\leftarrow\) 0,06 = \(\dfrac{6}{100}\)
Trường hợp 1 (nCO\(_2\) = 0,06 mol \(\Rightarrow\) nO\(_2\) dư = 0,03 mol)
Vậy mc = 0,06.12 = 0,75 (g)
VO\(_2\) = (0,06 + 0,03) . 22,4 = 2,016 (l)
Trường hợp 2 (nCO\(_2\) = 0,06 mol, nCO = \(\dfrac{1}{3}\) nCO\(_2\) = 0,02 mol)
\(\Rightarrow\) nC = nCO\(_2\) + nCO = 0,06 + 0,02 = 0,08 (mol)
\(\Rightarrow\) mC = 0,08 . 12 = 0,96 (g)
nO\(_2\) = nCO\(_2\) + \(\dfrac{1}{2}\) nCO = 0,06 + 0,01 = 0,07 (mol)
VO\(_2\) = 0,07.22,4 = 1,568 (l)
cho mình hỏi tại sao ở câu b th 1 no2 dư = 0,03 với còn th 2 thì nco = 1/3 nco2

\(n_C=\dfrac{1,2}{12}=0,1\left(mol\right)\)
PTHH: C + O2 --to--> CO2
a-->a--------->a
2C + O2 --to--> 2CO
b--->0,5b------>b
=> a + b = 0,1
Có: \(\overline{M}_X=\dfrac{44a+28b}{a+b}=16.2=32\)
=> a = 0,025; b = 0,075
\(n_{O_2}=a+0,5b=0,0625\left(mol\right)\)
=> \(V_{O_2}=0,0625.22,4=1,4\left(l\right)\)

a)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(n_{H_2O}=\dfrac{1,8}{18}=0,1\left(mol\right)\)
PTHH: 2H2 + O2 --to--> 2H2O
0,1<-0,05<-------0,1
2CO + O2 --to--> 2CO2
0,2<--0,1-------->0,2
=> \(\left\{{}\begin{matrix}V_{H_2}=0,1.22,4=2,24\left(l\right)\\V_{CO}=0,2.22,4=4,48\left(l\right)\end{matrix}\right.\)
b) \(m_{CO_2}=0,2.44=8,8\left(g\right)\)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15mol\)
\(n_{H_2O}=\dfrac{1,8}{18}=0,1mol\)
\(2CO+O_2\rightarrow2CO_2\)
a 0,5a a
\(2H_2+O_2\rightarrow2H_2O\)
0,1 0,05 \(\leftarrow\) 0,1
\(\Sigma n_{O_2}=0,5a+0,05=0,15\)
\(\Rightarrow a=n_{O_2\left(CO\right)}=0,2mol\)
\(V_{CO}=2\cdot0,2\cdot22,4=8,96l\)
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{CO_2}=0,2\cdot44=8,8g\)

\(n_{hhkhí}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Gọi \(n_{CO_2}=a\left(mol\right)\left(0< a< 0,25\right)\)
\(\rightarrow n_{CO}=0,25-a\left(mol\right)\)
Theo đề bài, ta có: \(\dfrac{44a+28\left(0,25-a\right)}{0,25}=17,2.2=34,4\left(\dfrac{g}{mol}\right)\)
\(\Leftrightarrow a=0,1\left(mol\right)\)
\(\rightarrow\left\{{}\begin{matrix}n_{CO_2}=0,1\left(mol\right)\\n_{CO}=0,25-0,1=0,15\left(mol\right)\end{matrix}\right.\)
PTHH:
C + O2 --to--> CO2
0,1 0,1
2C + O2 --to--> 2CO
0,15 0,15
=> mC = (0,1 + 0,15).12 = 3 (g)
=> B

a) \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,05-->0,1------->0,05
2C2H2 + 5O2 --to--> 4CO2 + 2H2O
0,125<--0,3125<----0,25
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,05}{0,05+0,125}.100\%=28,57\%\\\%V_{C_2H_2}=\dfrac{0,125}{0,05+0,125}.100\%=71,43\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,05.16}{0,05.16+0,125.26}.100\%=19,753\%\\\%m_{C_2H_2}=\dfrac{0,125.26}{0,05.16+0,125.26}.100\%=80,247\%\end{matrix}\right.\)
b) \(n_{O_2}=0,1+0,3125=0,4125\left(mol\right)\)
=> \(V_{O_2}=0,4125.22,4=9,24\left(l\right)\)
=> Vkk = 9,24.5 = 46,2 (l)

\(C+H_2O-^{^{ }t^{^{ }0}}->CO+H_2\\ C+2H_2O-^{^{ }t^{^0}}>CO_2+2H_2\\ m_X=11,2:22,4.7,8.2=7,8g\\ n_{CO}=a;n_{CO_2}=b\Rightarrow n_{H_2}=a+2b\left(mol\right)\\ n_X=0,5=a+b+a+2b=2a+3b=0,5\left(I\right)\\ m_X=28a+44b+2a+4b=30a+48b=7,8\left(II\right)\\ \left(I\right)\left(II\right)\Rightarrow a=0,1=b\\ n_{CO}=n_{CO_2}=0,1mol\\ n_{H_2}=0,3mol\)
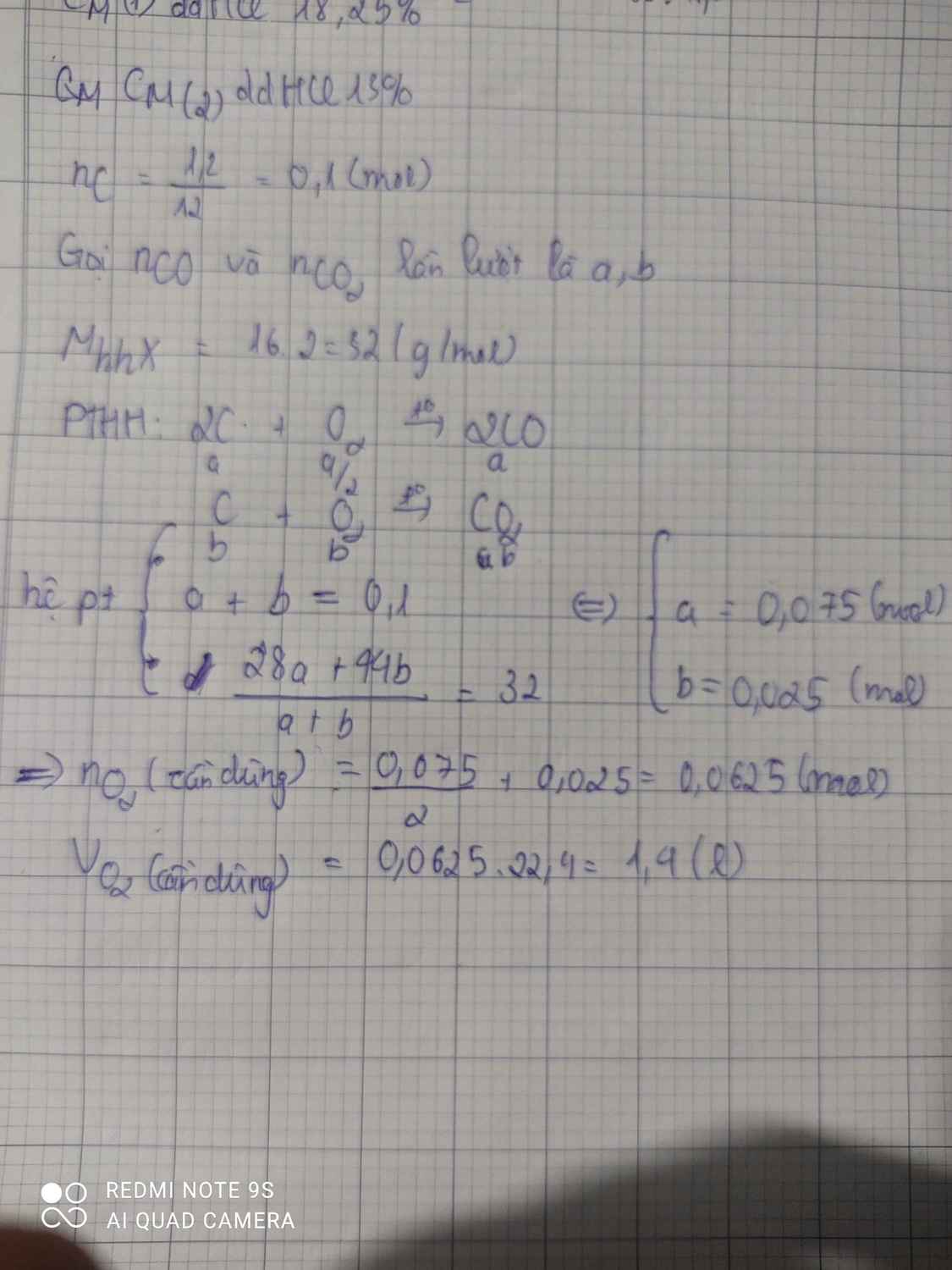
a) PTHH: \(C+O_2\xrightarrow[]{t^o}CO_2\)
\(C+\dfrac{1}{2}O_2\xrightarrow[]{t^o}CO\)
b) Ta có: \(\left\{{}\begin{matrix}\overline{M}=18,8\cdot2=37,6\\n_{khí}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\end{matrix}\right.\)
Theo sơ đồ đường chéo: \(\dfrac{n_{CO_2}}{n_{CO}}=\dfrac{37,6-28}{44-37,6}=\dfrac{3}{2}\) \(\Rightarrow\left\{{}\begin{matrix}n_{CO_2}=0,03\left(mol\right)\\n_{CO}=0,02\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{CO_2}=0,03\cdot22,4=0,672\left(l\right)\\V_{CO}=0,448\left(l\right)\end{matrix}\right.\)
c) Theo các PTHH: \(n_C=n_{CO_2}+n_{CO}=0,05\left(mol\right)\)
\(\Rightarrow m_C=0,05\cdot12=0,6\left(g\right)\)