Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Zn+\dfrac{1}{2}O_2\xrightarrow[]{t^o}ZnO\)
Bảo toàn khối lượng: \(m_{O_2}=m_{ZnO}-m_{Zn}=1,6\left(g\right)\)
a. \(2Zn+O_2\rightarrow2ZnO\)
b.\(m_{Zn}+m_{O_2}\rightarrow m_{ZnO}\)
\(\Rightarrow6,5+m_{O_2}=8,1\)
\(\Rightarrow m_O=8,1-6,5=1,6\)

\(n_{Fe_2O_3}=\dfrac{m}{M}=\dfrac{3,2}{160}=0,02mol\)
\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
0,02 0,06 0,04 ( mol )
\(V_{H_2}=n.22,4=0,06.22,4=1,334l\)
\(m_{Fe}=n.M=0,04.56=2,24g\)
nFe2O3 = 3,2/160 = 0,02 (mol)
PTHH: Fe2O3 + 3H2 -> (t°) 2Fe + 3H2O
Mol: 0,02 ---> 0,06 ---> 0,04
VH2 = 0,06 . 22,4 = 1,344 (l)
mFe = 0,04 . 56 = 2,24 (g)

a ) \(n_{Fe_2O_4}=\frac{23,2}{232}=0,1\) mol
\(Fe_3O_4+4H_2\underrightarrow{t^0}3Fe+4H_2O\)
0,1 -> 0,4 -> 0,3
\(\Rightarrow n_{H_2}=4n_{Fe_3O_4}=0,4\) mol \(\Rightarrow V_{H_2}=0,4.22,4=8,96\) lít
b ) \(n_{Fe}=3n_{Fe_3O_4}=0,3\) mol \(\Rightarrow m_{Fe}=56.0,3=16,8\) gam.

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{33,6}{56}=0,6\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,3\left(mol\right)\Rightarrow m_{Fe_2O_3}=0,3.160=48\left(g\right)\)
c, \(n_{H_2}=\dfrac{3}{2}n_{Fe}=0,9\left(mol\right)\Rightarrow V_{H_2}=0,9.22,4=20,16\left(l\right)\)

a) $4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3$
b) $n_{O_2} = \dfrac{6,72}{22,4} = 0,3(mol)$
$n_{Al\ pư} = \dfrac{4}{3}n_{O_2} = 0,4(mol)$
$m_{Al\ pư} = 0,4.27 = 10,8(gam)$
c)
Cách 1 :
$m_{Al_2O_3} = m_{Al} + m_{O_2} = 10,8 + 0,3.32 = 20,4(gam)$
Cách 2 :
Theo PTHH, $n_{Al_2O_3} = \dfrac{1}{2}n_{Al\ pư} = 0,2(mol)$
$m_{Al_2O_3} = 0,2.102 = 20,4(gam)$

\(n_{ZnO}=\dfrac{m}{M}=\dfrac{16,2}{65+16}=0,2\left(mol\right)\)
a) \(PTHH:Zn+H_2O\rightarrow ZnO+H_2\)
1 1 1 1
0,2 0,2 0,2 0,2
b) \(V_{H_2}=n.24,79=0,2.24,79=4,958\left(l\right)\)
c) \(m_{Zn}=n.M=0,2.65=13\left(g\right).\)
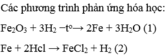
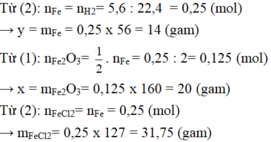

a/ \(3H_2+X_2O_3\rightarrow2X+3H_2O\)
b/ \(n_{H_2O}=\dfrac{m_{H_2O}}{M_{H_2O}}=\dfrac{2,7}{18}=0,15\left(mol\right)\)
Theo PTHH: \(n_{X_2O_3}=\dfrac{1}{3}n_{H_2O}=\dfrac{1}{3}.0,15=0,05\left(mol\right)\)
\(\Rightarrow M_{X_2O_3}=\dfrac{m_{X_2O_3}}{n_{X_2O_3}}=\dfrac{8}{0,05}=160\left(g/mol\right)\)
\(\Rightarrow2M_X+16.3=2M_X+48=160\)
\(\Leftrightarrow2M_X=160-48\)
\(\Leftrightarrow2M_X=112\)
\(\Leftrightarrow M_X=\dfrac{112}{2}=56\left(Fe\right)\)
Vậy kim loại đó là Fe
\(\Rightarrow CTHH:Fe_2O_3\)
c/ Theo PTHH: \(n_{Fe}=\dfrac{1}{2}n_{H_2O}=\dfrac{1}{2}0,15=0,075\left(mol\right)\)
Khối lượng kim loại tạo thành:
\(m_{Fe}=n_{Fe}.M_{Fe}=0,075.56=4,2\left(g\right)\)