Đốt cháy hoàn toàn 120g FeS2 trong KK a.Viết PTHH b.Tính VO2 (đktc) cần dùng biết O2 lấy dư 10% so vs lí thuyết c.Tính m sản phẩm thu đc nếu Hiệu suất H=80% Lớp 8
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


nFeS2 = 120/120 = 1 (mol)
PTHH: 4FeS2 + 11O2 -> (t°) 2Fe2O3 + 8SO2
Mol: 1 ---> 2,75 ---> 0,5 ---> 2
VO2 = 2,75/(100% - 10%) . 22,4 = 616/9 (l)
msp = (0,5 . 160 + 8 . 64) . 80% = 437,6 (g)

nFeS2 = 120/120 = 1 (mol)
PTHH: 4FeS2 + 11O2 -> (t°) 2Fe2O3 + 8SO2
Mol: 1 ---> 2,75 ---> 0,5 ---> 2
VO2 = 2,75/(100% - 10%) . 22,4 = 616/9 (l)
msp = (0,5 . 160 + 8 . 64) . 80% = 437,6 (g)

\(n_{C_2H_2}=\dfrac{2,6}{26}=0,1\left(mol\right)\\ 2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\\ a,n_{O_2}=\dfrac{5}{2}.n_{C_2H_2}=\dfrac{5}{2}.0,1=0,25\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\\ b,n_{CO_2}=\dfrac{4}{2}.n_{C_2H_2}=\dfrac{4}{2}.0,1=0,2\left(mol\right)\\ \Rightarrow m_{CO_2}=0.2.44=8,8\left(g\right)\\ n_{H_2O}=n_{C_2H_2}=0,1\left(mol\right)\\ \Rightarrow m_{H_2O}=0,1.18=1,8\left(g\right)\\ \Rightarrow m_{sp}=m_{CO_2}+m_{H_2O}=8,8+1,8=10,6\left(g\right)\)

\(a,n_{FeS_2}=\dfrac{m_{FeS_2}}{M_{FeS_2}}=\dfrac{6}{120}=0,05\left(mol\right)\\ 4FeS_2+11O_2\rightarrow\left(t^o,xt\right)2Fe_2O_3+8SO_2\uparrow\\ n_{Fe_2O_3}=\dfrac{2}{4}.n_{FeS_2}=\dfrac{2}{4}.0,05=0,025\left(mol\right)\\ \Rightarrow m_{Fe_2O_3}=160.0,025=4\left(g\right)\\ n_{SO_2}=\dfrac{8}{4}.n_{FeS_2}=\dfrac{8}{4}.0,05=0,1\left(mol\right)\\ \Rightarrow m_{SO_2}=0,1.64=6,4\left(g\right)\\ \Rightarrow m_{sp}=m_{Fe_2O_3}+m_{SO_2}=4+6,4=10,4\left(g\right)\\ b,n_{O_2}=\dfrac{11}{4}.n_{FeS_2}=\dfrac{11}{4}.0,05=0,1375\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,1375.22,4=3,08\left(l\right)\\ \Rightarrow V_{kk\left(đktc\right)}=3,08.5=15,4\left(l\right)\)
\(pthh:4FeS_2+11O_2\overset{t^o}{--->}2Fe_2O_3+8SO_2\uparrow\)
a. Ta có: \(n_{FeS_2}=\dfrac{6}{120}=0,05\left(mol\right)\)
Theo pt: \(n_{O_2}=\dfrac{11}{4}.n_{FeS_2}=\dfrac{11}{4}.0,05=0,1375\left(mol\right)\)
\(\Rightarrow m_{sản.phẩm.thu.được}=6+0,1375.32=10,4\left(g\right)\)
b. Ta có: \(V_{O_2}=0,1375.22,4=3,08\left(lít\right)\)
Mà: \(V_{O_2}=\dfrac{1}{5}V_{kk}\)
\(\Rightarrow V_{kk}=3,08.5=15,4\left(lít\right)\)

\(n_{hhk}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(n_{O_2}=\dfrac{26,88}{22,4}=1,2\left(mol\right)\)
Đặt \(\left\{{}\begin{matrix}n_{CH_4}=x\\n_{C_2H_4}=y\end{matrix}\right.\) ( mol ) \(\Rightarrow n_{hhk}=x+y=0,5\left(1\right)\)
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
x 2x ( mol )
\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
y 3y ( mol )
\(\rightarrow n_{O_2}=2x+3y=1,2\left(2\right)\)
\(\left(1\right);\left(2\right)\Rightarrow\left\{{}\begin{matrix}x=0,3\\y=0,2\end{matrix}\right.\)
\(\%V_{CH_4}=\dfrac{0,3}{0,5}.100=60\%\)
\(\%V_{C_2H_4}=100-60=40\%\)

\(n_{KClO_3}=\dfrac{24,5}{122,5}=0,2mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,2 0,2 0,3 ( mol )
\(V_{O_2}=0,3.24,79=7,437l\)
\(m_{KCl}=0,2.74,5=14,9g\)

X gồm: CH4 và C2H4
C2H4 + Br2 => C2H4Br2
mC2H4Br2 = m/M = 37.6/188 = 0.2 (mol)
Theo phương trình ==> nC2H4 = 0.2 (mol)
CH4 + 2O2 => CO2 + 2H2O (1)
C2H4 + 3O2 => 2CO2 + 2H2O (2)
nC2H4 = 0.2 (mol) => nCO2(2) = 0.6 (mol)
VCO2 = 16.8 (l) => nCO2 = 16.8/22.4 = 0.75 (mol)
==> nCO2 (1) = 0.75 - 0.6 = 0.15 (mol)
==> nCH4 = 0.075 (mol)
Ta có: nCH4 = 0.075 (mol), nC2H4 = 0.2 (mol)
===> nhh = 0.275 (mol)
%nCH4 = 27.27%, %nC2H4 = 72.73%
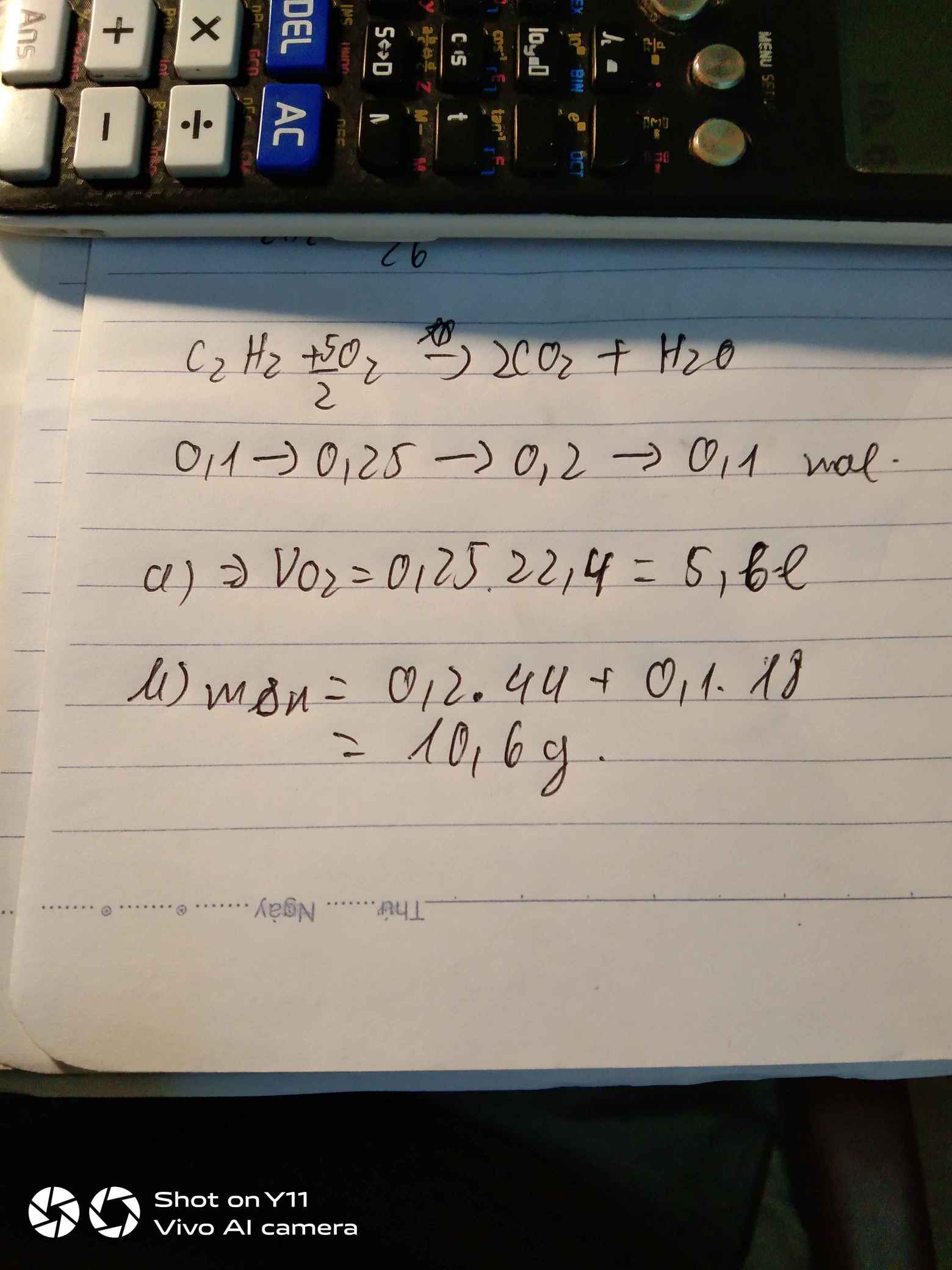
4FeS2+11O2-to>2Fe2O3+8SO2
1-------------2,75-------0,5-------2 mol
n FeS2=\(\dfrac{120}{120}=1mol\)
=>VO2=2,75.\(\dfrac{110}{100}\).32=96,8g
H=80%
=>m Fe2O3=0,5.160.\(\dfrac{80}{100}\)=64g
Có 100% mà hụt 10% thì sẽ là 100% - 10% = 90% nhé chị:)