Hòa tan 21,1 gam hỗn hợp A gồm Zn và ZnO bằng 200 gam dung dịch HCl ( vừa đủ ) thu được dung dịch B và 4,48 lít khí H2
a, Xác định khối lượng mỗi chất có trong hỗn hợp A
b, Tính C% của dung dịch HCl đã dùng
c, Tính khối lượng muối có trong dung dịch B
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
a) Ta có: \(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)=n_{Mg}\)
\(\Rightarrow\%m_{Mg}=\dfrac{0,5\cdot24}{16}=75\%\) \(\Rightarrow\%m_{MgO}=25\%\)
b) Ta có: \(\left\{{}\begin{matrix}n_{Mg}=0,5\left(mol\right)\\n_{MgO}=\dfrac{16\cdot25\%}{40}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{HCl}=2n_{Mg}+2n_{MgO}=1,2\left(mol\right)\) \(\Rightarrow m_{ddHCl}=\dfrac{1,2\cdot36,5}{20\%}=219\left(g\right)\)
c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{H_2}=0,5\left(mol\right)\\n_{MgCl_2}=0,6\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{H_2}=0,5\cdot2=1\left(g\right)\\m_{MgCl_2}=0,6\cdot95=57\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{hhA}+m_{ddHCl}-m_{H_2}=234\left(g\right)\) \(\Rightarrow C\%_{MgCl_2}=\dfrac{57}{234}\cdot100\%\approx24,36\%\)
Cho mình hỏi ở cái PTHH ấy! sao ta không tính số mol ở dưới??

PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(ZnO+2HCl\rightarrow ZnCl_2+H_2O\)
Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Theo PT: \(n_{Zn}=n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,2.65=13\left(g\right)\)
\(m_{ZnO}=21,1-13=8,1\left(g\right)\)
Có: \(n_{ZnO}=\dfrac{8,1}{81}=0,1\left(mol\right)\)
Theo PT: \(n_{HCl}=2n_{Zn}+2n_{ZnO}=0,6\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\Rightarrow C\%_{ddHCl}=\dfrac{21,9}{200}.100\%=10,95\%\)
Theo PT: \(n_{ZnCl_2}=n_{Zn}+n_{ZnO}=0,3\left(mol\right)\)
\(\Rightarrow m_{ZnCl_2}=0,3.136=40,8\left(g\right)\)
Bạn tham khảo nhé!
CcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCcccccccccccccccccccccCccccccccccccccccccccc

$a\big)$
$Zn+2CH_3COOH\to (CH_3COO)_2Zn+H_2$
$ZnO+2CH_3COOH\to (CH_2COO)_2Zn+H_2O$
Theo PT: $n_{Zn}=n_{H_2}=\frac{4,48}{22,4}=0,2(mol)$
$\to \%m_{Zn}=\frac{0,2.65}{21,1}.100\%\approx 61,61\%$
$\to \%m_{ZnO}=100-61,61=38,39\%$
$b\big)$
$n_{ZnO}=\frac{21,1-0,2.65}{81}=0,1(mol)$
Theo PT: $\sum n_{CH_3COOH}=2n_{Zn}+2n_{ZnO}=0,6(mol)$
$\to C_{M_{CH_3COOH}}=\dfrac{0,6}{\frac{200}{1000}}=3M$
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(Zn+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2\)
0,2 0,2 ( mol )
\(m_{Zn}=0,2.65=13g\)
\(\%m_{Zn}=\dfrac{13}{21,1}.100=61,61\%\)
\(\%m_{ZnO}=100\%-61,61\%=38,39\%\)
\(Zn+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2\)
0,2 0,4 ( mol )
\(n_{ZnO}=\dfrac{21,1-13}{81}=0,1mol\)
\(ZnO+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Zn+H_2O\)
0,1 0,2 ( mol )
\(C_{M\left(CH_3COOH\right)}=\dfrac{0,4+0,2}{0,2}=3M\)

2Al + 6HCl -> 2AlCl3 + 3H2 (1)
ZnO + 2HCl -> ZnCl2 + H2O (2)
a) nH2= 13,44/22.4=0.6(mol) -> mH2=0,6.2=1,2(g)
Theo PTHH: nAl = 2/3 nH2 = 2/3 . 0,6= 0,4(mol) -> mAl = 0,4 . 27=10,8(g)
-> mZnO = 27-10,8= 16,2(g)
b) nZnO = 16,2/81=0,2(mol)
Theo PTHH (2): nHCl = 2nZnO=2.0,2=0,4(mol)
Theo PTHH (1) : nHCl=2nH2=2.0,6=1,2(mol)
-> \(\Sigma\)nHCl = 0,4+1,2=1,6(mol)
-> mHCl = 1,6.36,5= 58,4(g)
-> mddHCl = 58,4.100/29,2= 200(g)
c) Theo PTHH (1): nAlCl3 = 2/3 nH2 = 2/3 . 0,6=0,4(mol) -> mAlCl3=0,4.133,5=53,4(g)
mdd sau phản ứng= mA + mddHCl - mH2 =27+200-1,2 =225,8(g)
-> C% AlCl3 = 53,4.100%/225,8 = 20,88%
Theo PTHH (2) nZnCl2 =nZnO= 0,2(mol)-> mZnCl2=0,2.136=27,2(g)
-> C% ZnCl2= 27,2.100%/255,8=10,63%

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo pt: \(\Rightarrow\left\{{}\begin{matrix}3x+y=0,2\\27x+56y=5,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=\dfrac{19}{470}\\y=\dfrac{37}{470}\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{5,5}\cdot100\%=19,84\%\)
\(\%m_{Fe}=100\%-19,84\%=80,16\%\)

a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
a_____2a______a_____a (mol)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
b_____3b_______b_____\(\dfrac{3}{2}\)b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}56a+27b=36,1\\a+\dfrac{3}{2}b=\dfrac{21,28}{22,4}=0,95\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,5\\b=0,3\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,5\cdot56=28\left(g\right)\\m_{Al}=8,1\left(g\right)\end{matrix}\right.\)
b+c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{HCl}=2a+3b=1,9\left(mol\right)\\n_{FeCl_2}=0,5\left(mol\right)\\n_{AlCl_3}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{HCl}}=\dfrac{1,9}{0,2}=9,5\left(M\right)\\C_{M_{FeCl_2}}=\dfrac{0,5}{0,2}=2,5\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\end{matrix}\right.\)

a) Gọi số mol Zn, Fe là a, b (mol)
=> 65a + 56b = 8,56 (1)
\(n_{H_2}=\dfrac{3,136}{22,4}=0,14\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
a--->2a-------->a----->a
Fe + 2HCl --> FeCl2 + H2
b----->2b------->b------>b
=> a + b = 0,14 (2)
(1)(2) => a = 0,08; b = 0,06
=> \(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{0,08.65}{8,56}.100\%=60,748\%\\\%m_{Fe}=\dfrac{0,06.56}{8,56}.100\%=39,252\%\end{matrix}\right.\)
b)
nKOH = 0,2.0,1 = 0,02 (mol)
PTHH: KOH + HCl --> KCl + H2O
0,02-->0,02
=> nHCl = 0,02 + 2a + 2b = 0,3 (mol)
=> \(C_{M\left(HCl\right)}=xM=\dfrac{0,3}{0,15}=2M\)
c) m = 0,08.136 + 0,06.127 = 18,5(g)
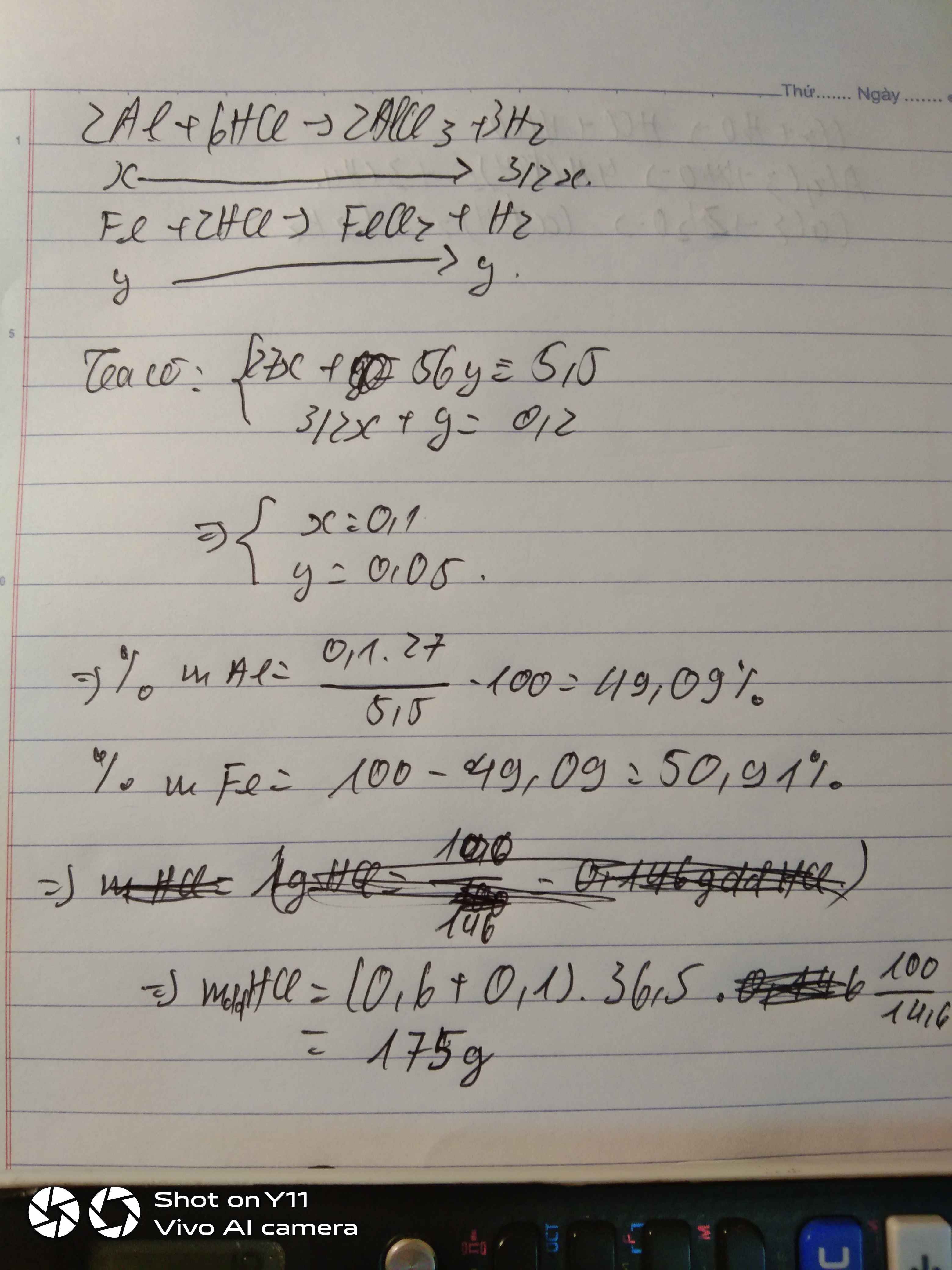
Zn+2HCl->ZnCl2+H2
ZnO+2HCl->ZnCl2+H2O
nH2=0.2(mol)->nZn=0.2(mol).mZn=13(g)
mZnO=21.1-13=8.1(g)
nZnO=0.1(mol)
Tổng nHCl cần dùng:0.2*2+0.1*2=0.6(mol)
mHCl=21.9(g)
C%ddHCl=21.9:200*100=10.95%
n muối=0.2+.1=0.3(mol)
m muối=40.8(g)