

Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


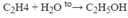

1) \(n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: C2H4 + H2O --axit--> C2H5OH
0,4-------------------->0,4
=> mC2H5OH = 0,4.46.70% = 12,88 (g)
\(V_{C_2H_5OH}=\dfrac{12,88}{0,8}=16,1\left(ml\right)\\ \rightarrowĐ_r=\dfrac{16,1}{50}.100=32,2^o\)
2) \(\left\{{}\begin{matrix}n_{C_2H_5OH}=\dfrac{36,8}{46}=0,8\left(mol\right)\\n_{CH_3COOH}=\dfrac{36}{60}=0,6\left(mol\right)\\n_{CH_3COOC_2H_5}=\dfrac{44}{88}=0,5\left(mol\right)\end{matrix}\right.\)
PTHH: CH3COOH + C2H5OH --H2SO4(đặc), to--> CH3COOC2H5 + H2O
0,5<-------------------------------------------------0,5
LTL: 0,6 < 0,8 => Hiệu suất phản ứng tính theo CH3COOH
=> \(H=\dfrac{0,5}{0,6}.100\%=83,33\%\)

`a)PTHH:`
`C_2 H_5 OH+K->C_2 H_5 OK+1/2H_2 \uparrow`
`K+H_2 O->KOH+1/2H_2 \uparrow`
`b)n_[H_2]=[7,84]/[22,4]=0,35(mol)`
Gọi `n_[C_2 H_5 OH]=x;n_[H_2 O]=y`
`=>` $\begin{cases} 46x+18y=21\\\dfrac{1}{2}x+\dfrac{1}{2}y=0,35 \end{cases}$
`<=>` $\begin{cases} x=0,3\\y=0,4 \end{cases}$
`@m_[C_2 H_5 OH]=0,3.46=13,8(g)`
`@m_[H_2 O]=21-13,8=7,2(g)`

a, \(CH_3COOH+Na\rightarrow CH_3COONa+\dfrac{1}{2}H_2\)
\(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
\(CH_3COOH+NaOH\rightarrow CH_3COONa+H_2O\)
b, Ta có: \(n_{NaOH}=0,2.0,5=0,1\left(mol\right)\)
Theo PT: \(n_{CH_3COOH}=n_{NaOH}=0,1\left(mol\right)\)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}+\dfrac{1}{2}n_{C_2H_5OH}=0,3\)
\(\Rightarrow n_{C_2H_5OH}=0,5\left(mol\right)\)
\(\Rightarrow m=m_{CH_3COOH}+m_{C_2H_5OH}=0,1.60+0,5.46=29\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CH_3COOH}=\dfrac{0,1.60}{29}.100\%\approx20,69\%\\\%m_{C_2H_5OH}\approx79,31\%\end{matrix}\right.\)

\(n_{NaOH}=0,2.1=0,2\left(mol\right)\\ a,CH_3COOH+NaOH\rightarrow CH_3COONa+H_2O\\ n_{CH_3COOH}=n_{NaOH}=0,2\left(mol\right)\\ b,m_{CH_3COOH}=0,2.60=12\left(g\right)\\ m_{C_2H_5OH}=20-12=8\left(g\right)\)