Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Ta có: \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{HCl}=2n_{H_2}=0,6\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\)
b, Theo PT: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Al}=0,2.27=5,4\left(g\right)\)

\(PTHH:2Al+6HCl->2AlCl_3+3H_2\)
0,2<--0,6<----------0,2<------0,3 (mol)
\(n_{H_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(m_{HCl}=n\cdot M=0,6\cdot\left(1+35,5\right)=21,9\left(g\right)\)
\(m_{AlCl_3}=n\cdot M=0,2\cdot\left(27+35,5\cdot3\right)=26,7\left(g\right)\)
a, PT: 2Al+6HCl→2AlCl3+3H2
Ta có: nH2=6,7222,4=0,3(mol)
Theo PT: nHCl=2nH2=0,6(mol)
⇒mHCl=0,6.36,5=21,9(g)
b, Theo PT: nAl=23nH2=0,2(mol)
⇒mAl=0,2.27=5,4(g)

Bài 1.
\(n_{CuO}=\dfrac{48}{80}=0,6mol\)
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,6 0,6 0,6 ( mol )
\(m_{Cu}=0,6.64=38,4g\)
\(V_{H_2}=0,6.22,4=13,44l\)
Bài 2.
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,25 0,5 0,25 ( mol )
\(m_{Fe}=0,25.56=14g\)
\(m_{HCl}=0,5.36,5=18,25g\)

\(n_{Fe}=\dfrac{m}{M}=\dfrac{28}{56}=0,5\left(mol\right)\\ PTHH:Fe+2HCl->FeCl_2+H_2\)
ti le 1 : 2 : 1 : 1
n(mol) 0,5-->1--------->0,5------>0,5
\(m_{FeCl_2}=n\cdot M=0,5\cdot\left(56+35,5\cdot2\right)=63,5\left(g\right)\\ V_{H_2\left(dktc\right)}=n\cdot22,4=0,5\cdot22,4=11,2\left(l\right)\)

\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,1<--0,1
=> mZnCl2 = 0,1.136 = 13,6 (g)

a)
\(PTHH:2Al+6HCl->2AlCl_3+3H_2\)
1,3<---4<-------1,3<---------2
b)
\(n_{H_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{44,8}{22,4}=2\left(mol\right)\)
\(m_{AlCl_3}=n\cdot M=1,3\cdot\left(27+35,5\cdot3\right)=173,55\left(g\right)\)
\(m_{Al}=n\cdot M=1,3\cdot27=35,1\left(g\right)\)

a: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b: \(n_{H2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(\Leftrightarrow n_{Al}=0.1\left(mol\right)\)
\(m_{Al}=n_{Al}\cdot M_{Al}=0.1\cdot27=2.7\left(g\right)\)
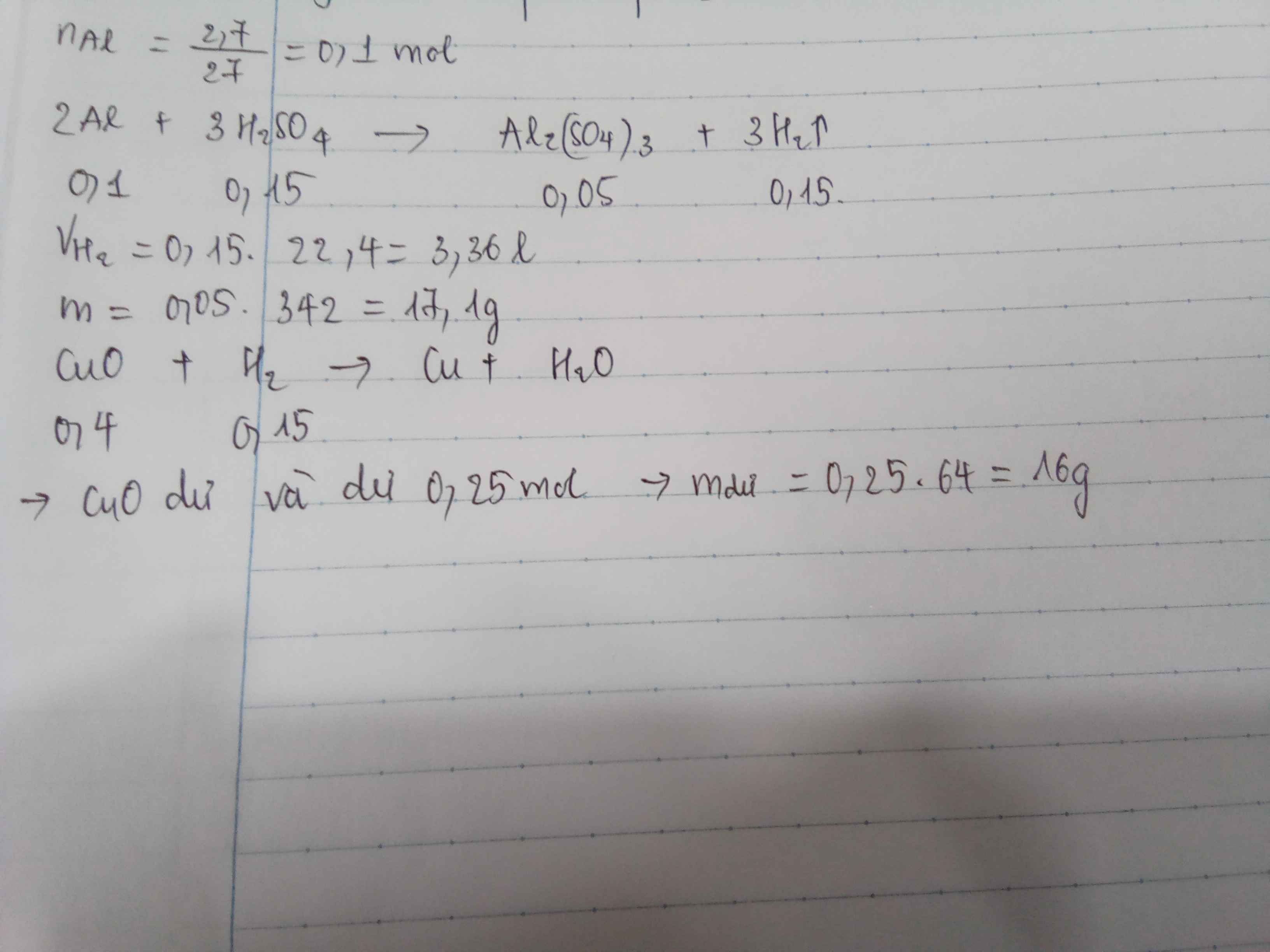
2Al + 6HCL → 2AlCl3 + 3H2
b) nH2 = 4,48 : 22,4= 0,2 mol => nAl = nAlCl3 = 0,2 : 3 . 2 = \(\dfrac{2}{15}\) mol
mAl = \(\dfrac{2}{15}\).27=3.6 g
mAlCl3 = \(\dfrac{2}{15}\)(27+35,5.3) = 17,8 g
Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
_____2/15___________2/15____0,2 (mol)
b, \(m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)
c, \(m_{AlCl_3}=\dfrac{2}{15}.133,5=17,8\left(g\right)\)
Bạn tham khảo nhé!