Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi số mol NaCl, NaI là a, b (mol)
=> 58,5a + 150b = 37,125 (1)
PTHH: 2NaI + Cl2 --> 2NaCl + I2
b------------>b
=> nNaCl(sau pư) = a + b = \(\dfrac{23,4}{58,5}=0,4\left(mol\right)\) (2)
(1)(2) => a = 0,25 (mol); b = 0,15 (mol)
=> \(\left\{{}\begin{matrix}\%m_{NaCl}=\dfrac{58,5.0,25}{37,125}.100\%=38,4\%\\\%m_{NaI}=\dfrac{0,15.150}{37,125}.100\%=60,6\%\end{matrix}\right.\)
=> A
Gọi \(\left\{{}\begin{matrix}n_{NaCl}=x\left(mol\right)\\n_{NaI}=y\left(mol\right)\end{matrix}\right.\)\(\Rightarrow58,5x+150y=37,125\left(1\right)\)
\(n_{NaCl}=\dfrac{23,4}{58,5}=0,4mol\)
\(\Rightarrow x+y=0,4\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,25mol\\y=0,15mol\end{matrix}\right.\)
\(\%m_{NaCl}=\dfrac{0,25\cdot58,5}{37,125}\cdot100\%=39,4\%\)
\(\%m_{NaI}=100\%-39,4\%=60,6\%\)
Chọn A

\(2NaBr+Cl_2\rightarrow2NaCl+Br_2\left(1\right)\\ m_{giảm}=m_{Br_2}-m_{Cl_2}\\ \Leftrightarrow n_{NaCl\left(1\right)}=n_{NaBr\left(1\right)}=\dfrac{13,35}{160-71}=0,15\left(mol\right)\\ \Rightarrow\%m_{NaBr}=\dfrac{103.0,15}{42,6}.100\approx36,268\%\\ \Rightarrow\%m_{NaCl}\approx63,732\%\)
Bổ sung:
\(C\%_{ddNaBr\left(trongA\right)}=\dfrac{0,15.103}{200}.100=7,725\%\\ C\%_{ddNaCl\left(trongA\right)}=\dfrac{42,6-0,15.103}{200}.100=13,575\%\)

\(Fe^{2+} \to Fe^{3+} + 1e\\ Mn^{+7} + 5e \to Mn^{2+}\\ \Rightarrow n_{Fe^{2+}} = 5n_{KMnO_4} = 0,18.5 =0,9(mol)\\ 2FeCl_3 + Fe \to 3FeCl_2\\ n_{FeCl_3} = \dfrac{2}{3}n_{FeCl_2} = 0,6(mol)\\ n_{Fe\ pư} = \dfrac{1}{3}n_{Fe} = 0,3(mol)\\ \Rightarrow m_{Fe\ trong\ A} = 2,8 + 0,3.56 = 19,6(gam)\\ 2Fe + 3Cl_2 \xrightarrow{t^o} 2FeCl_3\\ n_{Fe} = n_{FeCl_3} = 0,6(mol)\\\)
Phần trăm khối lượng Fe tham gia phản ứng là : \(\dfrac{0,6.56}{0,6.56 + 19,6}.100\% = 63,15\%\)
a ơi nhưng trong đề của cô e cho 4 đáp án không có đáp án 63,15%

\(FeS+2HCl\rightarrow FeCl_2+H_2S\)\(\uparrow\)
0.2 0.2
\(H_2S+4H_2O\rightarrow H_2SO_4+4H_2\)
0.2 0.2 0.8
a. \(n_{FeS}=\dfrac{17.6}{88}=0.2mol\)
\(mdd_{H_2SO_4}=m_X=m_{H_2S}+m_{H_2O}-m_{H_2}=0.2\times34+92.3-0.8\times2=97.5g\)
\(C\%_{H_2SO_4}=\dfrac{0.2\times98\times100}{97.5}=20,1\%\)
b. \(\dfrac{1}{2}dd_X\Rightarrow n_X=0.1mol\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
0.2 0.1
\(mdd_{NaOH}=\dfrac{0.2\times40\times100}{20}=40g\)

\(n_{HCl}=\dfrac{58,4.15\%}{36,5}=0,24\left(mol\right)\\ Fe+2HCl\rightarrow\left(t^o\right)FeCl_2+H_2\\ n_{Fe}=n_{H_2}=\dfrac{0,24}{2}=0,12\left(mol\right)\\ \Rightarrow V1=V_{H_2\left(đktc\right)}=0,12.22,4=2,688\left(l\right)\\ x=m_{Cu}=m_{hhA}-m_{Fe}=15,68-0,12.56=8,96\left(g\right)\\ b,n_{Cu}=\dfrac{8,96}{64}=0,14\left(mol\right)\\ 2Fe+3Cl_2\rightarrow\left(t^o\right)2FeCl_3\\ Cu+Cl_2\rightarrow\left(t^o\right)CuCl_2\\ n_{Cl_2}=\dfrac{3}{2}.n_{Fe}+n_{Cu}=\dfrac{3}{2}.0,12+0,14=0,32\left(mol\right)\\ \Rightarrow V2=V_{Cl_2\left(đktc\right)}=0,32.22,4=7,168\left(l\right)\\ y=m_{muối}=m_{AlCl_3}+m_{CuCl_2}=0,12.133,5+0,14.135=34,92\left(g\right)\)

\(Đặt:n_{Mg}=a\left(mol\right);n_{Al}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\\ 2Al+6HCl\rightarrow2AlCl_3+3H_2\\ \Rightarrow\left\{{}\begin{matrix}24a+27b=5,1\\22,4a+22,4.1,5.b=5,6\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\\ a,\Rightarrow\%m_{Mg}=\dfrac{0,1.24}{5,1}.100\approx47,059\%\\ \Rightarrow\%m_{Al}\approx100\%-47,059\%\approx52,941\%\\ b,n_{HCl}=2.n_{H_2}=2.\left(0,1+0,1.1,5\right)=0,5\left(mol\right)\\ \Rightarrow V_{ddHCl}=\dfrac{0,5}{2}=0,25\left(l\right)\)
a)\(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
x 2x x x
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
y 3y y 1,5y
Ta có hệ:
\(\left\{{}\begin{matrix}24x+27y=5,1\\x+1,5y=\dfrac{5,6}{22,4}=0,25\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,1\end{matrix}\right.\)
\(\%m_{Mg}=\dfrac{0,1\cdot24}{5,1}\cdot100\%=47,06\%\)
\(\%m_{Al}=100\%-47,06\%=52,94\%\)
b)\(\Sigma n_{HCl}=2x+3y=2\cdot0,1+3\cdot0,1=0,5mol\)
\(V=\dfrac{n}{C_M}=\dfrac{0,5}{2}=0,25l=250ml\)

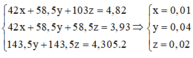
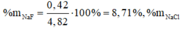
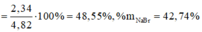
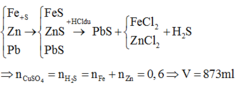
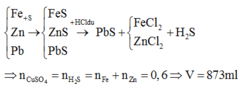
\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Pt : \(2NaCl+2H_2O\xrightarrow[có.màng.ngăn]{điện.phân}2NaOH+H_2+Cl_2|\)
2 2 2 1 1
0,6 0,3
\(2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3|\)
2 3 2
0,2 0,3
\(n_{NaCl}=\dfrac{0,3.2}{1}=0,6\left(mol\right)\)
⇒ \(m_{NaCl}=0,6.58,5=35,1\left(g\right)\)
Chúc bạn học tốt