Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) PTPU

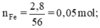
Theo pt: nH2 = nFe = 0,05 (mol)
VH2 = 22,4.n = 22,4.0,05 = 1,12 (l)
b) nHCl = 2.nFe = 2. 0,05 = 0,1 (mol)
mHCl = M.n = 0,1.36,5 = 3,65 (g)

\(a,PTHH:Fe+2HCl\rightarrow FeCl_2+H_2\)
\(n_{Fe}=\frac{m_{Fe}}{M_{Fe}}=\frac{1,4}{56}=0,025\left(mol\right)\)
\(\Rightarrow n_{HCl}=0,05\left(mol\right)\)
\(m_{HCl}=M.n=0,05.36,5=1,825\left(g\right)\)
\(b,n_{H_2}=n_{Fe}=0,025\)
\(\Rightarrow V_{H_2}=n.22,4=0,025.22,4=0,56\left(l\right)\)

a) \(PTHH:Fe+HCL\) → \(FeCl_2+H_2\)
Cân bằng: \(Fe+2HCl\) → \(FeCl_2+H_2\)
b) \(n_{Fe}=\dfrac{m}{M}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
\(n_{HCl}=2.n_{Fe}=2.0,1=0,2\left(mol\right)\)
\(m_{HCl}=n.M=0,2.36,5=7,3\left(g\right)\)
c) \(n_{Fe}=n_{H_2}=0,1\left(mol\right)\)
\(V_{H_2\left(đktc\right)}=n.22,4=0,1.22,4=2,24\left(l\right)\)

a)
\(PTHH:Mg+2HCl->MgCl_2+H_2\)
2<------4<----------2<---------2 (mol)
b)
\(n_{H_2\left(dktc\right)}=\dfrac{V}{22,4}=\dfrac{44,8}{22,4}=2\left(mol\right)\)
\(m_{HCl}=n\cdot M=4\cdot\left(1+35,5\right)=146\left(g\right)\)
c)
\(m_{MgCl_2}=n\cdot M=2\cdot\left(24+71\right)=190\left(g\right)\)
\(n_{H_2}=\dfrac{V}{22,4}=\dfrac{44,8}{22,4}=2\left(mol\right)\)
\(\text{a)}Mg+2HCl\rightarrow MgCl_2+H_2\)
\(2mol\) \(1mol\) \(1mol\)
\(4mol\) \(2mol\) \(2mol\)
\(b)m_{HCl}=n.M=4.36,5=146\left(g\right)\)
\(c)m_{MgCl_2}=n.M=2.95=190\left(g\right)\)

1/ Fe +2 HCl --------> FeCl2 + H2
\(n_{Fe}=\dfrac{2,8}{56}=0,05\left(mol\right)\)
\(n_{H_2}=n_{Fe}=0,05\left(mol\right)\Rightarrow V_{H_2}=0,05.22,4=1,12\left(l\right)\)
\(n_{HCl}=2n_{Fe}=0,1\left(mol\right)\Rightarrow m_{HCl}=0,1.36,5=3,65\left(g\right)\)
Câu 6 :
1) $n_{Fe} = \dfrac{2,8}{56} = 0,05(mol)$
Fe + 2HCl → FeCl2 + H2
0,05...0,1....................0,05......(mol)$
$V_{H_2} = 0,05.22,4 = 1,12(lít)$
$m_{HCl} = 0,1.36,5 = 3,65(gam)$
2)
a) $CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O$
$V_{O_2} = 2V_{CH_4} = 4(lít)$
b) $n_{CO_2} = n_{CH_4} = 0,15(mol) \Rightarrow V_{CO_2} = 0,15.22,4 = 3,36(lít)$
c) $d_{CH_4/kk} = \dfrac{16}{29} = 0,552$
Vậy khí metan nhẹ hơn không khí 0,552 lần
\(n_{Fe}=\dfrac{1.4}{56}=0.025\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.025.....0.05...............0.025\)
\(m_{HCl}=0.05\cdot36.5=1.825\left(g\right)\)
\(V_{H_2}=0.025\cdot22.4=0.56\left(l\right)\)