
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(1.a.2Mg+O_2-^{t^o}\rightarrow2MgO\\ b.Fe+2AgNO_3\rightarrow Fe\left(NO_3\right)_2+2Ag\\ c.C_2H_4+3O_2-^{t^o}\rightarrow2CO_2+2H_2O\\ d.CuO+2HCl\rightarrow CuCl_2+H_2O\\ e.2Na+2H_2O\rightarrow2NaOH+H_2\\ f.4Al+3O_2-^{t^o}\rightarrow2Al_2O_3\)
\(2.a.Magie+Axitclohidric\rightarrow MagieClorua+Hidro\\ b.Mg+2HCl\rightarrow MgCl_2+H_2\\ c.m_{Mg}+m_{HCl}=m_{MgCl_2}+m_{H_2}\\ d.m_{HCl}=m_{MgCl_2}+m_{H_2}-m_{Mg}=47,5+1-12=36,5\left(g\right)\)

\(n_{Fe_3O_4}=\dfrac{8}{232}=\dfrac{1}{29}\left(mol\right)\)
\(Fe_3O_4+8HCl\rightarrow FeCl_2+2FeCl_3+4H_2O\)
\(\dfrac{1}{29}.......\dfrac{8}{29}................\dfrac{2}{29}\)
\(m_{HCl}=\dfrac{8}{29}\cdot36.5=10.06\left(g\right)\)
\(m_{FeCl_3}=\dfrac{2}{29}\cdot162.5=11.21\left(g\right)\)

Câu 5 :
a)
$n_{NaOH} = 0,1.2,5 = 0,25(mol)$
$m_{NaOH} = 0,25.40 = 10(gam)$
b)
$C_{M_{CaCl_2}} = \dfrac{0,02}{0,2} = 0,1M$
c)
$C\%_{NaCl} = \dfrac{6}{6 + 144}.100\% = 4\%$

2.
a) 4Cr + 3O2 → 2Cr2O3 (4:3:2)
b) 2Fe + 3Br2 → 2FeBr3 (2:3:2)
c) 2KClO3 → 2KCl + 3O2 (2:2:3)
d) 2NaNO3 → 2NaNO2 + O2 (2:2:1)
e) Zn + 2HCl → ZnCl2 + H2 (1:2:1:1)
g) S + O2 → SO2 (1:1:1)
f) 4P + 5O2 → 2P2O5 (4:5:2)
i) 3Fe + 2O2 → Fe3O4 (3:2:1)
k) CH4 + 2O2 → CO2 + 2H2O (1:2:1:2)
m) 2NaOH + H2SO4 → Na2SO4 + 2H2O (2:1:1:2)
n) BaCl2 + 2AgNO3 →2AgCl + Ba(NO3)2 (1:2:2:1)
s) Fe + 2AgNO3 → Fe(NO3)2 + 2Ag(1:2:1:2)
t) 2Al(OH)3 → Al2O3 + 3H2O (2:1:3)
3. a) 2C2H2 + 5O2 → 4CO2 + 2H2O (2:5:4:2)
b) 2Al + 6HCl → 2AlCl3 + 3H2 (2:6:2:3)

nC = \(\dfrac{6}{12}=0,5\left(mol\right)\)
\(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: C + O2 ---to---> CO2.
Ta thấy: \(\dfrac{0,5}{1}>\dfrac{0,1}{1}\)
=> Cacbon dư
Theo PT: \(n_{CO_2}=n_{O_2}=0,1\left(mol\right)\)
=> \(V_{CO_2}=0,1.22,4=2,24\left(lít\right)\)

a. CnH2n + \(\dfrac{3n}{2}\)O2 ---> nCO2 + nH2O
b. CnH2n + 2 + \(\dfrac{3n+1}{2}\)O2 ---> nCO2 + (n + 1)\(\)H2O
c. CxHy + (4x + y)O2 ---> xCO2 + \(\dfrac{y}{2}\)H2O

\(a.C_3H_8+5O_2\underrightarrow{^{to}}3CO_2+4H_2O\)
Đó anh viết PT cho em , em tự tính nha, đề không cho số liệu mà.

\(n_{CH_4}=\dfrac{3,2}{16}=0,2\left(mol\right)\)
PTHH: CH4 + 2O2 ---to→ CO2 + 2H2O
Mol: 0,2 0,4
\(V_{O_2}=0,4.22,4=8,96\left(l\right)\)

 mình đang cần gấp mng giúp mình nhé
mình đang cần gấp mng giúp mình nhé
 Giúp mình với mình đang cần rất gấp , vì mình đang thi
Giúp mình với mình đang cần rất gấp , vì mình đang thi
 giúp mình với mình đang cần gấp
giúp mình với mình đang cần gấp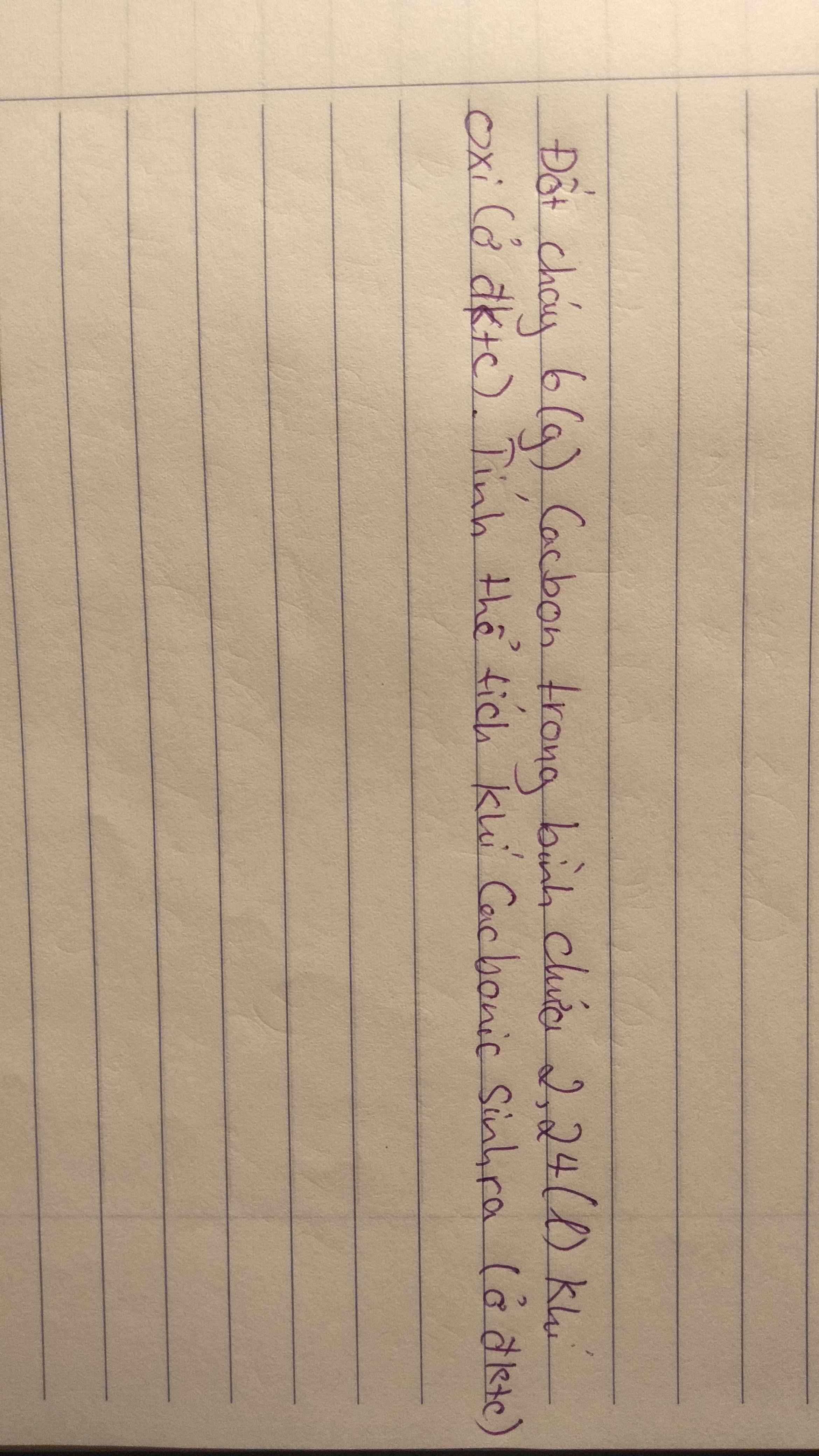

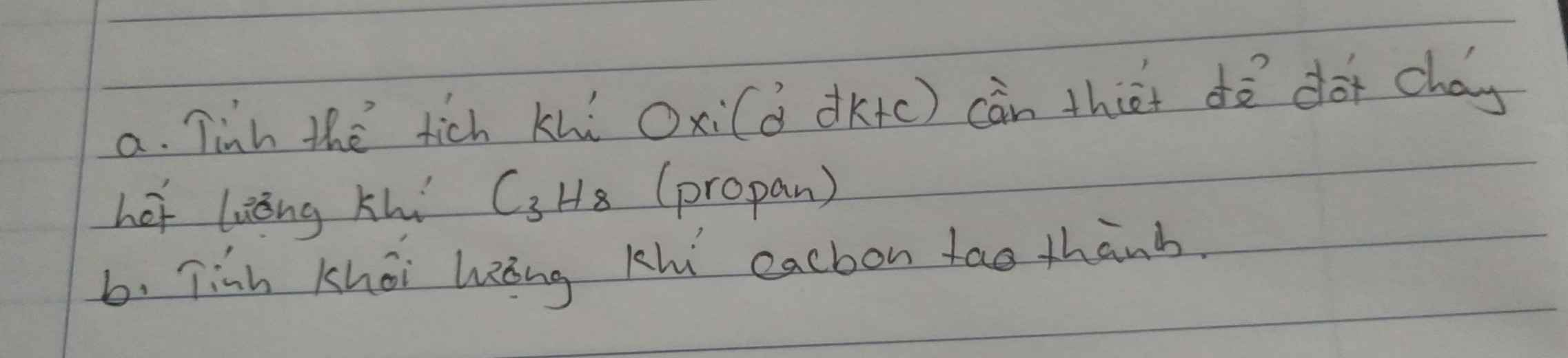


thi :>
Bạn đang thi nên 45p nữa mình giúp nhé:)