
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



a) \(m_O=\dfrac{20.20}{100}=4\left(g\right)\)
=> \(n_{CaO}=n_O=\dfrac{4}{16}=0,25\left(mol\right)\)
\(\left\{{}\begin{matrix}\%m_{CaO}=\dfrac{0,25.56}{20}.100\%=70\%\\\%m_{Ca}=100\%-70\%=30\%\end{matrix}\right.\)
b) \(n_{Ca}=\dfrac{20.30\%}{40}=0,15\left(mol\right)\)
PTHH: Ca+ 2H2O --> Ca(OH)2 + H2
0,15-------------------->0,15
=> V = 0,15.22,4 = 3,36 (l)
\(n_{Fe_3O_4}=\dfrac{23,2}{232}=0,1\left(mol\right)\)
=> nFe = 0,3 (mol)
=> mFe = 0,3.56 = 16,8 (g)
=> \(m=\dfrac{16,8.100}{78,9474}=21,28\left(g\right)\)
c) Giả sử Fe3O4 bị khử thành Fe
Gọi số mol Fe3O4 pư là a (mol)
PTHH: Fe3O4 + 4H2 --> 3Fe + 4H2O
a--->4a----->3a
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,15}{4}\) => Hiệu suất tính theo H2
m = 23,2 - 232a + 168a = 21,28
=> a = 0,03 (mol)
=> \(\left\{{}\begin{matrix}n_{Fe_3O_4\left(pư\right)}=0,03\left(mol\right)\\n_{H_2\left(pư\right)}=0,12\left(mol\right)\end{matrix}\right.\)
\(H=\dfrac{n_{H_2\left(pư\right)}}{n_{H_2\left(bđ\right)}}=\dfrac{0,12}{0,15}.100\%=80\%\)

Câu 1 :
$M + O_2 \xrightarrow{t^o} MO_2$
Theo PTHH : $n_M = n_{MO_2} \Rightarrow \dfrac{2}{M} = \dfrac{2,54}{M + 32}$
$\Rightarrow M = 118,5 \to$ Sai đề
Câu 2 :
$2Ba + O_2 \xrightarrow{t^o} 2BaO$
$n_{O_2} = \dfrac{1}{2}n_{BaO} = 0,5.\dfrac{36,72}{153} = 0,12(mol)$
$4Fe(NO_3)_3 \xrightarrow{t^o} 2Fe_2O_3 + 12NO_2 + 3O_2$
$n_{Fe(NO_3)_3} = \dfrac{4}{3}n_{O_2} = 0,16(mol)$
$m_{Fe(NO_3)_3} = 0,16.242 = 38,72(gam)$

Câu 1 : Gọi $n_C = a(mol) ; n_S = b(mol)$
Ta có : $12a + 32b = 5,6(1)$
$C + O_2 \xrightarrow{t^o} CO_2$
$S + O_2 \xrightarrow{t^o} SO_2$
$n_{O_2} = a + b = 0,3(2)$
Từ (1)(2) suy ra a = 0,2 ; b = 0,1
$m_C = 0,2.12 = 2,4(gam) ; m_S = 0,1.32 = 3,2(gam)$
Câu 2 :
$4R + 3O_2 \xrightarrow{t^o} 2R_2O_3$
Theo PTHH : $n_R = 2n_{R_2O_3} \Rightarrow \dfrac{2,16}{R} = 2.\dfrac{4,08}{2R + 16.3}$
$\Rightarrow R = 27$
Vậy R là nhôm, KHHH : Al
Câu 3 :
1 tấn= 1000 kg
$m_{Fe_3O_4} = 1000.90\% = 900(kg)$
$n_{Fe_3O_4} = \dfrac{900}{232}(kmol)$
$n_{Fe} = 3n_{Fe_3O_4} = \dfrac{900}{232}.3 = \dfrac{675}{58}(kmol)$
$m_{Fe} = \dfrac{675}{58}.56 = 651,72(kg)$

\(PTHH:CH_4+2O_2\underrightarrow{t^o}2H_2O+CO_2\)
\(n_{CH_4}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(Theo.PTHH:n_{O_2}=2.n_{CH_4}=2.0,05=0,1\left(mol\right)\\ V_{O_2\left(đktc\right)}=n.22,4=0,1.22,4=2,24\left(l\right)\\ \\ Theo.PTHH:n_{CO_2}=n_{CH_4}=0,05\left(mol\right)\\ V_{CO_2}=n.22,4=0,05.22,4=1,12\left(l\right)\)

m NaCl = 200.11,6% = 23,2(gam)
$2NaCl + 2H_2O \xrightarrow{đpdd} 2NaOH + H_2 + Cl_2$
n H2 = 1,68/22,4 = 0,075(mol)
Theo PTHH :
n NaCl pư = n NaOH = 2n H2 = 0,15(mol)
n Cl2 = n H2 = 0,075(mol)
Sau phản ứng :
m NaCl = 23,2 - 0,15.58,5 = 14,425(gam)
m dd = 200 - 0,075.2 - 0,075.71 = 194,525(gam)
Ta có :
C% NaCl = 14,425/194,525 .100% = 7,42%
C% NaOH = 0,15.40/194,525 .100% = 3,08%

a, Gọi CTHH X là \(R_2H_6\)
Ta có \(PTK_{R_2H_6}=2\cdot NTK_R+6=PTK_{NO}=14+16=30\left(đvC\right)\)
Do đó \(NTK_R=12\left(đvC\right)\)
Vậy R là cacbon (C)
b, \(\%m_R=\dfrac{12\cdot2}{30}\cdot100\%=80\%\)
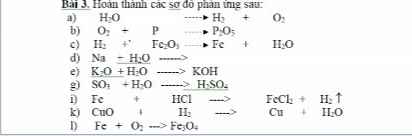
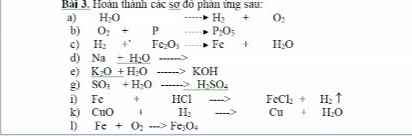









Bài 3
a) \(H_2O-^{đpdd}\rightarrow H_2+\dfrac{1}{2}O_2\)
b) \(5O_2+4P-^{t^o}\rightarrow2P_2O_5\)
c) \(3H_2+Fe_2O_3\rightarrow2Fe+3H_2O\)
d) \(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
e) \(K_2O+H_2O\rightarrow2KOH\)
g) \(SO_3+H_2O\rightarrow H_2SO_4\)
i) \(Fe+2HCl\rightarrow FeCl_2+H_2\)
k) \(CuO+H_2-^{t^o}\rightarrow Cu+H_2O\)
l) \(3Fe+2O_2-^{t^o}\rightarrow Fe_3O_4\)