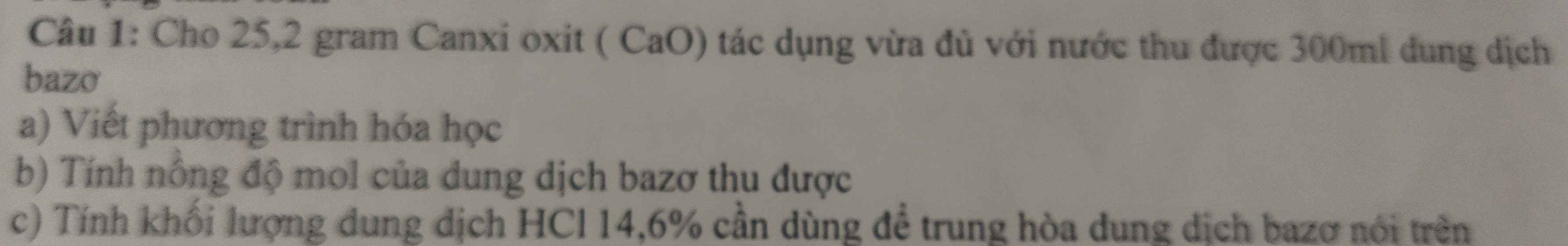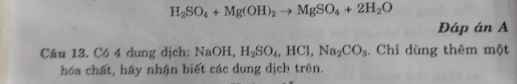Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Ta có: \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
\(a.PTHH:\)
\(Mg+2HCl--->MgCl_2+H_2\left(1\right)\)
\(CuO+2HCl--->CuCl_2+H_2O\left(2\right)\)
b. Theo PT(1): \(n_{Mg}=n_{H_2}=0,25\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,25.24=6\left(g\right)\)
\(\Rightarrow m_{CuO}=24,25-6=18,25\left(g\right)\)
c. Ta có: \(n_{CuO}=\dfrac{18,25}{80}=\dfrac{73}{320}\left(mol\right)\)
\(\Rightarrow n_{hh}=\dfrac{73}{320}+0,25=0,478125\left(mol\right)\)
Theo PT(1,2): \(n_{HCl}=2.n_{hh}=2.0,478125=0,95625\left(mol\right)\)
Đổi 300ml = 0,3 lít
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,95625}{0,3}=3,1875M\)



a) \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
_____0,2<-----------------0,2<--0,2
=> \(\%Mg=\dfrac{0,2.24}{10}.100\%=48\%\)
b) \(C\%\left(MgCl_2\right)=\dfrac{0,2.95}{0,2.24+200-0,2.2}.100\%=9,3\%\)

\(n_{NaOH}=\dfrac{12}{40}=0.3\left(mol\right)\)
\(n_{HCl}=\dfrac{7.3}{36.5}=0.2\left(mol\right)\)
\(NaOH+HCl\rightarrow NaCl+H_2O\)
Ta có :
\(n_{NaOH}>n_{HCl}\Rightarrow NaOHdư\)
\(n_{NaOH\left(pư\right)}=n_{HCl}=n_{NaCl}=0.2\left(mol\right)\)
\(n_{NaOH\left(dư\right)}=0.3-0.2=0.1\left(mol\right)\)
\(m_{cr}=m_{NaOH\left(dư\right)}+m_{NaCl}=0.1\cdot40+0.2\cdot58.5=15.7\left(g\right)\)
Ta có: \(n_{NaOH}=\dfrac{12}{40}=0,3\left(mol\right)\)
\(n_{HCl}=\dfrac{7,3}{36,5}=0,2\left(mol\right)\)
PTHH: NaOH + HCl ---> NaCl + H2O
Ta thấy: \(\dfrac{0,3}{1}>\dfrac{0,2}{1}\)
Vậy NaOH dư, HCl hết.
Theo PT: \(n_{NaCl}=n_{HCl}=0,2\left(mol\right)\)
\(\Rightarrow m_{NaCl}=0,2.58,5=11,7\left(g\right)\)



a) Gọi x, y lần lượt là số mol Al, Fe
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Fe + H2SO4 → FeSO4+ H2
\(\left\{{}\begin{matrix}27x+56y=5,54\\\dfrac{3}{2}x+y=\dfrac{3,584}{22,4}\end{matrix}\right.\)
=> x=0,06 , y =0,07
=> \(m_{Al}=1,62\left(g\right);m_{Fe}=3,92\left(g\right)\)
b) \(n_{H_2SO_4\left(pứ\right)}=n_{H_2}=0,16\left(mol\right)\)
=> \(m_{H_2SO_4\left(pứ\right)}=0,16.98=15,68\left(g\right)\)
c) \(m_{ddH_2SO_4}=\dfrac{15,68}{20\%}=78,4\left(g\right)\)
c) 2NaOH + H2SO4 → Na2SO4 + 2H2O
\(n_{H_2SO_4\left(dư\right)}=\dfrac{1}{2}n_{NaOH}=\dfrac{1}{2}.0,25.0,6=0,075\left(mol\right)\)
=> \(m_{H_2SO_4\left(bđ\right)}=15,68+0,075.98=23,03\left(g\right)\)