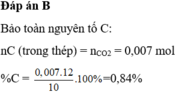Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(C+O_2\xrightarrow[]{t^o}CO_2\)
Tính theo sản phẩm
Ta có: \(n_C=n_{CO_2}=\dfrac{264}{44}=6\left(mol\right)\)
\(\Rightarrow\%m_C=\dfrac{12\cdot6}{120}\cdot100\%=60\%\) \(\Rightarrow\%_{tạp.chất}=40\%\)
\(n_{CO_2}=\frac{264}{44}=6(mol)\\ C+O_2\buildrel{{t^o}}\over\longrightarrow CO_2\\ n_{C}=n_{CO_2}=6(mol)\\ m_{C}=6.12=72(g)\\ \%m_{\text{tạp chât}}=\frac{120-72}{120}.100\%=40\%\)

nKNO3 = 0.2 (mol)
2KNO3 -to-> 2KNO2 + O2
0.2__________0.2____0.1
mKNO2 = 0.2*85=17(g)
C + O2 -to-> CO2
x___x
S + O2 -to-> SO2
y___y
x + y = 0.1 (1)
Mặt khác :
+) 32y/m * 100% = 4%
=> 32y = 0.04m (2)
+) 12x/m = 0.92
=> 12x = 0.92m (3)
(1) , (2) , (3) :
x = 0.1
y = 0.002
m = 1.3
VCO2 + VSO2 = ( 0.1 + 0.002) * 22.4 = 2.2848 (l)

\(n_{O_2}=\dfrac{8}{32}=0.25\left(mol\right)\)
\(CH_4+2O_2\underrightarrow{t^0}CO_2+2H_2O\)
\(0.125....0.25....0.125\)
\(m_{CH_4}=0.125\cdot16=2\left(g\right)\)
\(V_{CO_2}=0.125\cdot22.4=2.8\left(l\right)\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
\(............0.125.....0.125\)
\(m_{CaCO_3}=0.125\cdot100=12.5\left(g\right)\)

a, \(CH_4+2O_2\underrightarrow{t^o}CO_2+H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
Ta có: \(n_{CH_4}+n_{C_2H_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\left(1\right)\)
Theo PT: \(n_{CO_2}=n_{CH_4}+2n_{C_2H_2}=\dfrac{56}{22,4}=2,5\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{CH_4}=0,5\left(mol\right)\\n_{C_2H_2}=1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,5.22,4}{33,6}.100\%\approx33,33\%\\\%V_{C_2H_2}\approx66,67\%\end{matrix}\right.\)
b, Theo PT: \(n_{O_2}=2n_{CH_4}+\dfrac{5}{2}n_{C_2H_2}=3,5\left(mol\right)\Rightarrow m_{O_2}=3,5.32=112\left(g\right)\)

\(C+O_2=CO_2\)
375 375 375(mol)
\(\Rightarrow m_{CO_2}=375.44=16500\left(g\right)=16,5kg\)
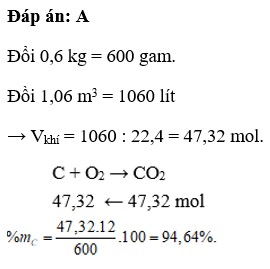
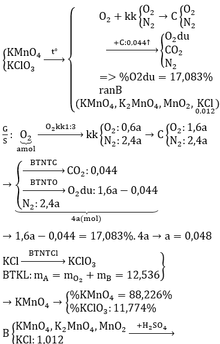
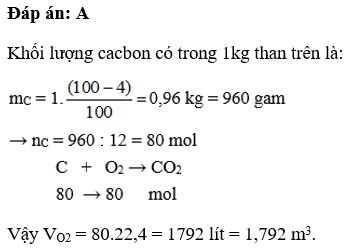
⇒A