Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{CH_4}=a\left(mol\right),n_{C_2H_2}=b\left(mol\right)\)
\(\Rightarrow m_A=16a+26b=8.4\left(g\right)\left(1\right)\)
\(n_{O_2}=\dfrac{28.8}{32}=0.9\left(mol\right)\)
\(CH_4+2O_2\underrightarrow{t^0}CO_2+2H_2O\)
\(C_2H_2+\dfrac{5}{2}O_2\underrightarrow{t^0}2CO_2+H_2O\)
\(\Rightarrow2a+\dfrac{3}{2}b=0.9\left(2\right)\)
\(\left(1\right),\left(2\right):a=b=0.2\)
\(m_{CH_4}=0.2\cdot16=3.2\left(g\right)\)
\(m_{C_2H_2}=0.2\cdot26=5.2\left(g\right)\)
Chúc em học tốt !!
Sory anh lại sai đề là CH4 và C2H2 mới đúng. Ko phải C2H4. Phiền a giải lại ạ.

a) PTHH: \(2CO+O_2\underrightarrow{t^o}2CO_2\) (1)
\(4H_2+O_2\underrightarrow{t^o}2H_2O\) (2)
b) Ta có: \(\left\{{}\begin{matrix}\Sigma n_{O_2}=\dfrac{9,6}{32}=0,3\left(mol\right)\\n_{CO_2}=\dfrac{8,8}{44}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{O_2\left(1\right)}=0,1mol\\n_{O_2\left(2\right)}=0,2mol\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CO}=0,1\cdot28=2,8\left(g\right)\\m_{H_2}=0,2\cdot2=0,4\left(g\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{CO}=\dfrac{2,8}{2,8+0,4}\cdot100\%=87,5\%\\\%m_{H_2}=12,5\%\end{matrix}\right.\)
c) PTHH: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
Theo PTHH: \(n_{KMnO_4}=2n_{O_2}=0,6mol\)
\(\Rightarrow m_{KMnO_4}=0,6\cdot158=94,8\left(g\right)\)

CH4 + 2O2 t0→ CO2 + 2H2O
C2H4 + 3O2 t0→ 2CO2 + 2H2O
C2H2 + 52 O2 t0→ 2CO2 + 2H2O
-Gọi: nCH4:a(mol)
nC2H4:b(mol)
nC2H2:c(mol)
⇒16a+28b+26c=11(1)
BTNT C ⇒a+2b+2c=0,75(2)
-Phân tích (1)và (2) ta được:
{13a+26b+26c=9,75
=>3a+2b=1,25(3)
16a+32b+32c=12
=>4b+6c=1(4)
-Từ (3) ⇒ \(a=\dfrac{1,25-2b}{3}\)
-Từ (4)⇒\(\dfrac{1-4c}{6}\)
-% CH4 =\(\dfrac{16a}{16a+32b+32c}.100\)
-Thay công thức a và c vào (⋅)
⇒%CH4=\(\dfrac{\dfrac{1,25-2b}{3}16}{16\dfrac{1,25-2b}{3}+28b+26.\dfrac{1-4b}{6}}100=12,12\%\)

\(2CO + O_2 \xrightarrow{t^o} 2CO_2\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ n_{CO} = n_{CO_2} = \dfrac{8,8}{44} = 0,2(mol)\\ n_{O_2} = \dfrac{n_{CO} + n_{H_2}}{2}=\dfrac{0,2+n_{H_2}}{2} = \dfrac{9,6}{32} = 0,3(mol)\\ \Rightarrow n_{H_2} = 0,4(mol)\\ \%V_{CO} = \dfrac{0,2}{0,2 + 0,4}.100\% = 33,33\%\\ \%V_{H_2} = 100\% - 33,33\% = 66,67\%\\ \%m_{CO} = \dfrac{0,2.28}{0,2.28+0,4.2}.100\%=87,5\%\\ \%m_{H_2} = 100\% - 87,5\% = 12,5\%\)

a, \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
b, Sửa đề: 17,9 (l) → 17,92 (l)
Ta có: \(n_{CO_2}=\dfrac{17,92}{22,4}=0,8\left(mol\right)=n_C\)
\(n_{H_2O}=\dfrac{18}{18}=1\left(mol\right)\Rightarrow n_H=1.2=2\left(mol\right)\)
⇒ mA = mC + mH = 0,8.12 + 2.1 = 11,6 (g)
Theo ĐLBT KL, có: mA + mO2 = mCO2 + mH2O
⇒ mO2 = 0,8.44 + 18 - 11,6 = 41,6 (g)
\(\Rightarrow n_{O_2}=\dfrac{41,6}{32}=1,3\left(mol\right)\Rightarrow V_{O_2}=1,3.22,4=29,12\left(l\right)\)

\(n_{CH_4}=\dfrac{2}{16}=0,125\left(mol\right)\)
\(PTHH:CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(\left(mol\right)\) \(0,125\) \(0,25\)
Đặt \(\left\{{}\begin{matrix}n_{KMnO_4}=a\left(mol\right)\\n_{KClO_3}=b\left(mol\right)\end{matrix}\right.\)
\(\%m_K=26,68\left(\%\right)\Leftrightarrow\dfrac{39\left(a+b\right)}{158a+122,5b}=\dfrac{26,68}{100}\)
Lại có: \(0,5a+1,5b=0,25\) ( Cái này viết PTHH ra mới thấy)
\(\Rightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{KMnO_4}=72\left(\%\right)\\\%m_{KClO_3}=28\left(\%\right)\end{matrix}\right.\)
$CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O$
$n_{O_2} = 2n_{CH_4} = 2.\dfrac{2}{16} = 0,25(mol)$
Gọi $n_{KMnO_4} = a(mol) ; n_{KClO_3} = b(mol)$
Ta có :
$\dfrac{39(a + b)}{158a + 122,5b} = \dfrac{26,68}{100}(1)$
$2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2$
$2KClO_3 \xrightarrow{t^o} 2KCl + 3O_2$
$n_{O_2} = 0,5a + 1,5b = 0,25(2)$
Từ (1)(2) suy ra a = 0,19 ; b = 0,1$
Ta có :
$\%m_{KMnO_4} = \dfrac{0,19.158}{0,19.158 + 0,1.122,5}.100\% = 71\%$
$\%m_{KClO_3} = 100\% - 71\% = 29\%$

\(a,Đặt:n_{CH_4}=a\left(mol\right);n_{C_4H_{10}}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\\ 2C_4H_{10}+13O_2\rightarrow\left(t^o\right)8CO_2+10H_2O\\ \Rightarrow\left\{{}\begin{matrix}16a+58b=7,4\\22,4a+22,4.4b=22\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{CH_4}=0,1.16=1,6\left(g\right)\\m_{C_4H_{10}}=0,1.58=5,8\left(g\right)\end{matrix}\right.\\ b,n_{O_2}=2a+\dfrac{13}{2}b=2.0,1+6,5.0,1=0,85\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,85.22,4=19,04\left(l\right)\)
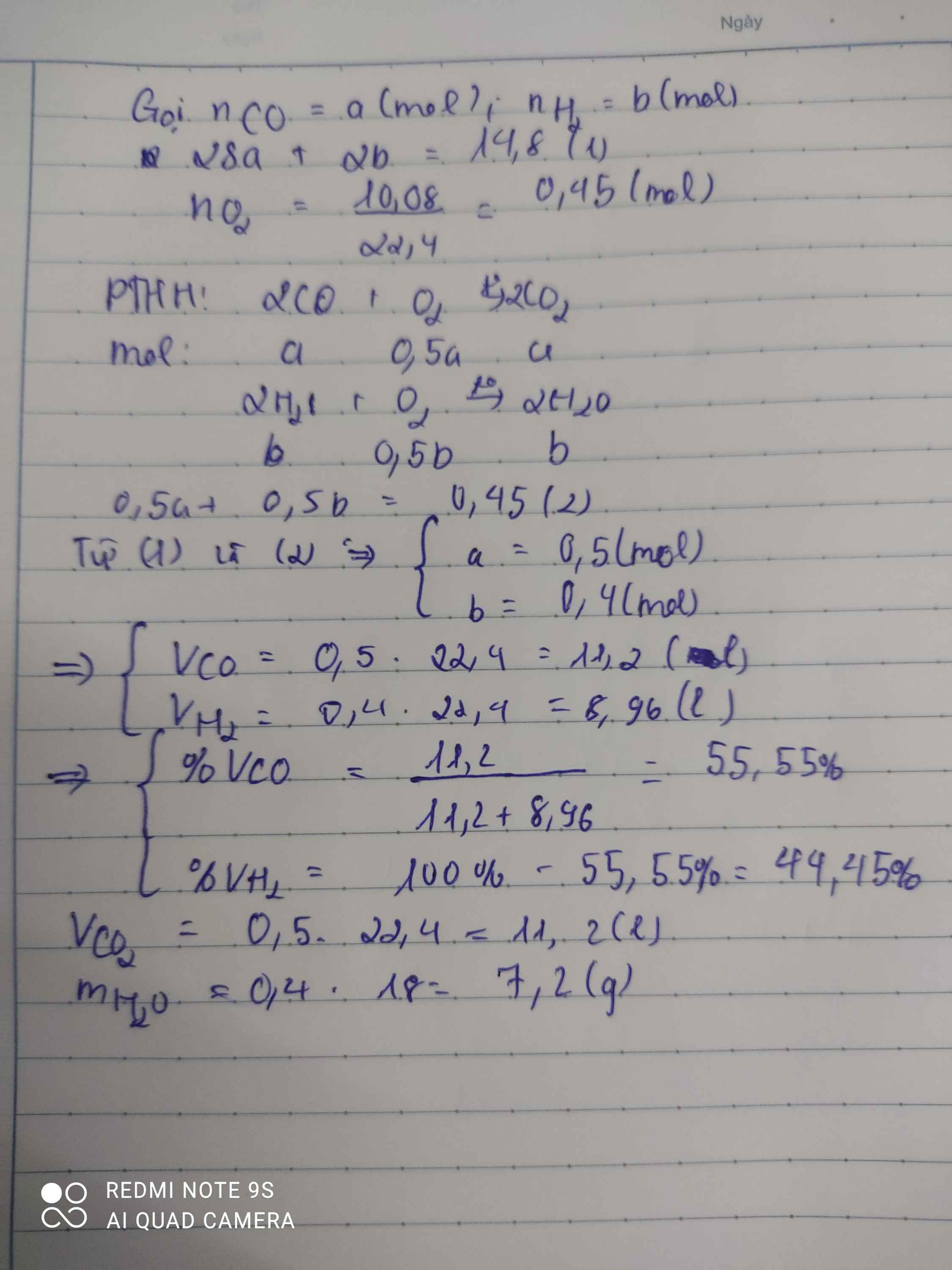
\(m_{CH_4} = 8.30\% = 2,4(gam)\\ m_{C_2H_4} = 8 - 2,4 = 5,6(gam)\\ CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ C_2H_4 + 3O_2 \xrightarrow{t^o} 2CO_2 + 2H_2O\\\)
Theo PTHH :
\(n_{CO_2} = n_{CH_4} + 2n_{C_2H_4} = \dfrac{2,4}{16} + \dfrac{5,6}{28}.2 = 0,55(mol)\\ \Rightarrow m_{CO_2} = 0,55.44 = 24,2(gam)\)
\(m_{CH_4}=0.3\cdot8=2.4\left(g\right)\)
\(n_{CH_4}=\dfrac{2.4}{16}=0.15\left(mol\right)\)
\(m_{C_2H_4}=8-2.4=5.6\left(g\right)\)
\(n_{C_2H_4}=\dfrac{5.6}{28}=0.2\left(mol\right)\)
\(CH_4+2O_2\underrightarrow{t^0}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^0}2CO_2+2H_2O\)
\(n_{CO_2}=2\cdot0.15+0.2=0.5\left(mol\right)\)
\(m_{CO_2}=0.5\cdot44=22\left(g\right)\)