Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

S + O2 \(\xrightarrow[]{t^o}\) SO2
nS = 1,6/32 = 0,05 mol
Theo pt: nO2 = nS = 0,05 mol
=> VO2 = 0,05.22,4 = 1,12 lít

a)
\(m_{MgCl_2}=\dfrac{50.4}{100}=2\left(g\right)\Rightarrow m_{H_2O}=50-2=48\left(g\right)\)
b)
\(n_S=\dfrac{6,4}{32}=0,2\left(mol\right)\)
PTHH: S + O2 --to--> SO2
0,2->0,2
=> VO2 = 0,2.22,4 = 4,48 (l)
=> Vkk = 4,48 : 20% = 22,4 (l)
a.\(m_{MgCl_2}=\dfrac{50.4}{100}=2g\)
\(m_{H_2O}=50-2=48g\)
b.\(n_S=\dfrac{6,4}{32}=0,2mol\)
\(S+O_2\rightarrow\left(t^o\right)SO_2\)
0,2 0,2 ( mol )
\(V_{kk}=V_{O_2}.5=\left(0,2.22,4\right).5=22,4l\)

\(n_C=\dfrac{3.6}{12}=0.3mol\)
\(C+O_2\underrightarrow{t^o}CO_2\)
0.3 0.3
\(V_{O_2}=0.3\times22.4=6.72l\)
\(V_{Kk}=6.72\times5=33.6l\)

\(a,PTHH:S+O_2\underrightarrow{t^o}SO_2\left(1\right)\)
\(n_S=\dfrac{m}{M}=\dfrac{9,6}{32}=0,3\left(mol\right)\)
\(Theo.PTHH\left(1\right):n_O=n_S=0,3\left(mol\right)\)
\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\\ Theo.PTHH\left(2\right):n_{KClO_3}=\dfrac{2}{3}.n_{O_2}=\dfrac{2}{3}.0,3=0,2\left(mol\right)\\ m_{KClO_3}=n.M=0,2.122,5=24,5\left(g\right)\)
\(b,V_{O_2\left(đktc\right)}=n.22,4=0,2.22,4=4,48\left(l\right)\\ \Rightarrow V_{kk\left(đktc\right)}=5.V_{O_2}=5.4,48=22,4\left(l\right)\)

\(n_S=\dfrac{3.2}{32}=0.1\left(mol\right)\)
\(n_{O_2}=\dfrac{1.12}{22.4}=0.05\left(mol\right)\)
\(S+O_2\underrightarrow{t^0}SO_2\)
\(0.05.0.05...0.05\)
\(\Rightarrow Sdư\)
\(V_{SO_2}=0.05\cdot22.4=1.12\left(l\right)\)
\(b.\)
\(S+O_2\underrightarrow{t^0}SO_2\)
\(0.1..0.1\)
\(V_{kk}=5V_{O_2}=5\cdot0.1\cdot22.4=11.2\left(l\right)\)
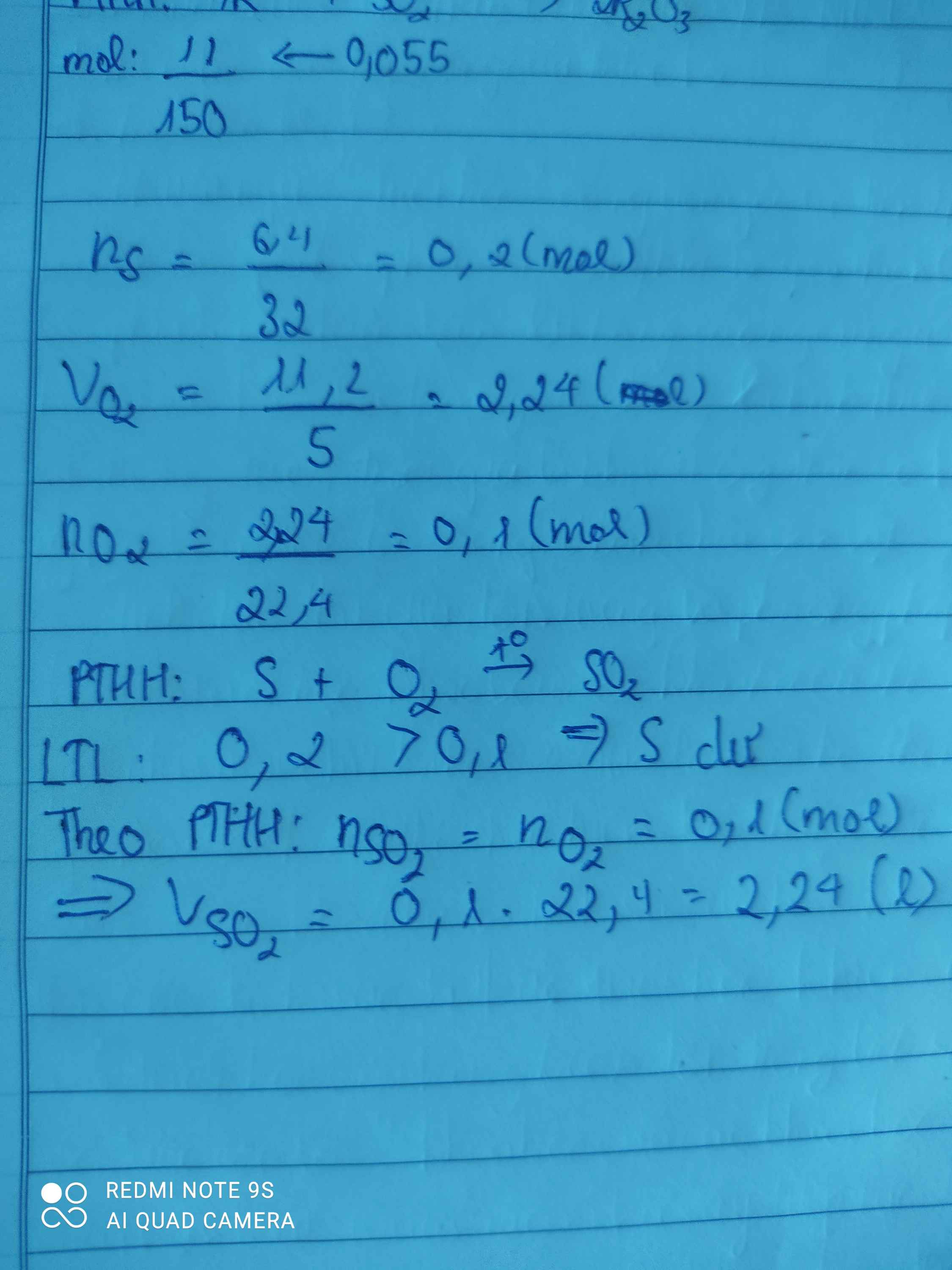
\(n_S=\dfrac{6.4}{32}=0,2\left(mol\right)\)
PTHH : S + O2 ---to---> SO2
0,2 0,2
\(V_{O_2}=0,2.22,4=4,48\left(l\right)\)
\(V_{kk}=4,48.5=22,4\left(l\right)\)
B