Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
$Zn + S \xrightarrow{t^o} ZnS$
$n_{Zn} =\dfrac{9,75}{65} = 0,15 > n_S = \dfrac{3,84}{32} = 0,12$ nên Zn dư
$n_{ZnS} = n_S = 0,12(mol)$
$m_{ZnS} = 0,12.97 = 11,64(gam)$
$n_{Zn\ dư} = 0,15 - 0,12 = 0,03(mol)$
$m_{Zn\ dư} = 0,03.65 = 1,95(gam)$
b)
$Zn + 2HCl \to ZnCl_2 + H_2$
$ZnS + 2HCl \to ZnCl_2 + H_2S$
$n_{khí} = n_{H_2} + n_{H_2S} = n_{Zn\ dư} + n_{ZnS} = 0,15(mol)$
$V = 0,15.22,4 = 3,36(lít)$
\(n_{Zn}=\dfrac{9.75}{65}=0.15\left(mol\right)\)
\(n_S=\dfrac{3.84}{32}=0.12\left(mol\right)\)
\(Zn+S\underrightarrow{^{^{t^0}}}ZnS\)
Lập tỉ lệ :
\(\dfrac{0.15}{1}>\dfrac{0.12}{1}\Rightarrow Zndư\)
\(a.\)
\(m_X=m_{ZnS}+m_{Zn\left(dư\right)}=0.12\cdot97+\left(0.15-0.12\right)\cdot65=13.59\left(g\right)\)
\(b.\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.03..................................0.03\)
\(ZnS+2HCl\rightarrow ZnCl_2+H_2S\)
\(0.12.................................0.12\)
\(V_{khí}=0.03\cdot22.4+0.12\cdot22.4=3.36\left(l\right)\)

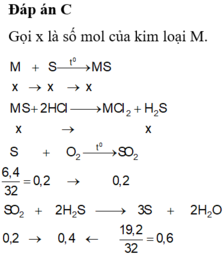
Chọn đáp án D
n H C l = 2 n O o x i t = 2 3 , 43 - 2 , 15 16 =0,16 (mol)
=> V d d H C l = 0 , 16 0 , 5 = 0,32 (lít)

BTKL mO=44,6-28,6=16 --> nO=0,5
BTe nBr=2nO=0,5.2=1 --> 11,2l
M=mBr + mhh= 42,9+0,5.160=122,9g

a) Đặt \(\left\{{}\begin{matrix}n_{Fe}=a\left(mol\right)\\n_{Al}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow56a+27b=1,93\) (1)
Ta có: \(n_S=\dfrac{1,28}{32}=0,04\left(mol\right)\)
Bảo toàn electron: \(2a+3b=0,08\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=\dfrac{121}{3800}\left(mol\right)\\b=\dfrac{31}{5700}\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=\dfrac{121}{3800}\cdot56\approx1,78\left(g\right)\\m_{Al}\approx0,15\left(g\right)\end{matrix}\right.\)
b) Bảo toàn nguyên tố: \(\Sigma n_{H_2S}=n_{FeS}+3n_{Al_2S_3}=n_{Fe}+6n_{Al}=\dfrac{49}{760}\left(mol\right)\)
\(\Rightarrow V_{H_2S}=\dfrac{49}{760}\cdot22,4\approx1,44\left(l\right)\)

a) Chất rắn không tan là Cu
=> m Cu = 19,2(gam)
n Mg = a(mol) ; n Fe = b(mol)
=> 24a + 56b = 32,8 -19,2 = 13,6(1)
$Mg + H_2SO_4 \to MgSO_4 + H_2$
$Fe + H_2SO_4 \to FeSO_4 + H_2$
n H2 = a + b = 6,72/22,4 = 0,3(2)
Từ (1)(2) suy ra a = 0,1 ; b = 0,2
%m Cu = 19,2/32,8 .100% = 58,54%
%m Mg = 0,1.24/32,8 .100% = 7,32%
%m Fe = 100% -58,54% -7,32% = 34,14%
b)
m dd A = 32,8 + 200 - 0,3.2 = 232,2(gam)
n MgSO4 = a = 0,1(mol)
n FeSO4 = b = 0,2(mol)
C% MgSO4 = 0,1.120/232,2 .100% = 5,17%
C% FeSO4 = 0,2.152/232,2 .100% = 13,09%

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Zn}=y\left(mol\right)\end{matrix}\right.\)
\(n_{HCl}=0,2\cdot4=0,8mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(x\) \(\rightarrow\) \(3x\) \(x\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(y\) \(\rightarrow\) \(2y\) \(y\)
\(\Rightarrow\left\{{}\begin{matrix}27x+65y=11,9\\3x+2y=0,8\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
a)\(\%m_{Al}=\dfrac{0,2\cdot27}{11,9}\cdot100\%=45,38\%\)
\(\%m_{Zn}=100\%-45,38\%=54,62\%\)
b)\(\Sigma n_{H_2}=\dfrac{3}{2}x+y=\dfrac{3}{2}\cdot0,2+0,1=0,4mol\)
\(V_{H_2}=0,4\cdot22.4=8,96l\)