Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) PTHH: \(4Fe+3O_2\underrightarrow{t^o}2Fe_2O_3\)
Ta có: \(n_{Fe}=\dfrac{m}{M}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
PTPƯ: 4Fe + 3O2 \(\underrightarrow{t^o}\) 2Fe2O3
4 3 2
0,3 0,225 0,15
\(\Rightarrow V_{O_2}=n.24,79=0,225.24,79=5,57775\left(l\right)\)

10.
\(n_{Fe}=\dfrac{16.8}{56}=0.3\left(mol\right)\)
\(4Fe+3O_2\underrightarrow{^{^{t^0}}}2Fe_2O_3\)
\(0.3.....0.225....0.15\)
\(V_{O_2}=0.225\cdot22.4=5.04\left(l\right)\)
\(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
\(0.15...........0.45\)
\(m_{H_2SO_4}=0.45\cdot98=44.1\left(g\right)\)
11.
\(n_{Fe_2O_3}=\dfrac{48}{160}=0.3\left(mol\right)\)
\(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
\(0.3...........1.8...........0.6\)
\(m_{FeCl_3}=0.6\cdot162.5=97.5\left(g\right)\)
\(m_{HCl}=1.8\cdot36.5=65.7\left(g\right)\)
Bài 10:
\(a,n_{Fe}=\dfrac{16,8}{56}=0,3(mol)\\ PTHH:4Fe+3O_2\xrightarrow{t^o}2Fe_2O_3\\ Fe_2O_3+3H_2SO_4\to Fe_2(SO_4)_3+3H_2O\\ \Rightarrow n_{O_2}=\dfrac{3}{4}n_{Fe}=0,225(mol)\\ \Rightarrow V_{O_2}=0,225.22,4=5,04(l)\\ b,n_{H_2SO_4}=3n_{Fe_2O_3}=3.\dfrac{1}{2}n_{Fe}=0,45(mol)\\ \Rightarrow m_{H_2SO_4}=0,45.98=44,1(g)\)
Bài 11:
\(a,n_{Fe_2O_3}=\dfrac{48}{160}=0,3(mol)\\ PTHH:Fe_2O_3+6HCl\to 2FeCl_3+3H_2O\\ \Rightarrow n_{FeCl_3}=2n_{Fe_2O_3}=0,6(mol)\\ \Rightarrow m_{FeCl_3}=0,6.162,5=97,5(g)\\ b,n_{HCl}=6n_{Fe_2O_3}=1,8(mol)\\ \Rightarrow m_{HCl}=1,8.36,5=65,7(g)\)

Bài 1 :
\(2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2\\ n_{O_2} = \dfrac{1}{2}n_{KMnO_4} = \dfrac{1}{2}.\dfrac{47,4}{158} = 0,15(mol)\\ 3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4\\ n_{Fe_3O_4} = \dfrac{1}{2}n_{O_2} = 0,075(mol)\\ m_{Fe_3O_4} = 0,075.232 = 17,4(gam)\)

2O2+3Fe-to>Fe3O4
Fe3O4+4H2-to>3Fe+4H2O
2Fe+3Cl2-to>2Fecl3
Fe+2HCl->Fecl2+H2
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
\(Fe_3O_4+4H_2\rightarrow\left(t^o\right)3Fe+4H_2O\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)

\(1) Fe_2O_3 + 3H_2 \xrightarrow{t^o} 2Fe + 3H_2O\\ Fe_3O_4 + 4H_2 \xrightarrow{t^o} 3Fe + 4H_2O \text{Theo PTHH }\\ n_{H_2O} = n_{H_2} = \dfrac{20,16}{22,4}=0,9(mol)\\ \text{Bảo toàn khối lượng : }\\ a = m_{hh} + m_{H_2} - m_{H_2O} = 65,4 + 0,9.2 - 0,9.18 = 51(gam)\)
2)
\(n_{Mg} = a ; n_{Al} = b ; n_{Fe} = c\\ \Rightarrow 24a + 27b + 56c = 18,6(1)\\ Mg + 2HCl \to MgCl_2 + H_2\\ 2Al + 6HCl \to 2AlCl_3 + 3H_2\\ Fe + 2HCl \to FeCl_2 + H_2\\ n_{H_2} = a + 1,5b + c = \dfrac{14,56}{22,4}=0,65(2)\\ 2Mg + O_2 \xrightarrow{t^o} 2MgO\\ 4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3\\ 3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4\\ n_{O_2} = \dfrac{7,84}{22,4} = 0,35\)
Ta có :
\(\dfrac{a + b + c}{0,5a + 0,75b + \dfrac{2}{3}c} = \dfrac{0,55}{0,35}(3)\\ (1)(2)(3) \Rightarrow a = 0,2 ; b = 0,2 ; c= 0,15\\ \%m_{Mg} = \dfrac{0,2.24}{18,6}.100\% = 25,81\%\\ \%m_{Al} = \dfrac{0,2.27}{18,6}.100\% = 29,03\%\\ \%m_{Fe} = 100\% - 25,81\% -29,03\% = 45,16\%\)

\(a,4K+O_2\rightarrow2K_2O\\ K_2O+H_2O\rightarrow2KOH\\ b,4P+3O_{2\left(thiếu\right)}\rightarrow2P_2O_3\\ P_2O_3+3H_2O\rightarrow2H_3PO_3\\ c,3Fe+2O_2\rightarrow\left(t^o\right)3Fe_3O_4\\ Fe_3O_4+8Al\rightarrow\left(t^o\right)9Fe+4Al_2O_3\\ Fe+2HCl\rightarrow FeCl_2+H_2\)
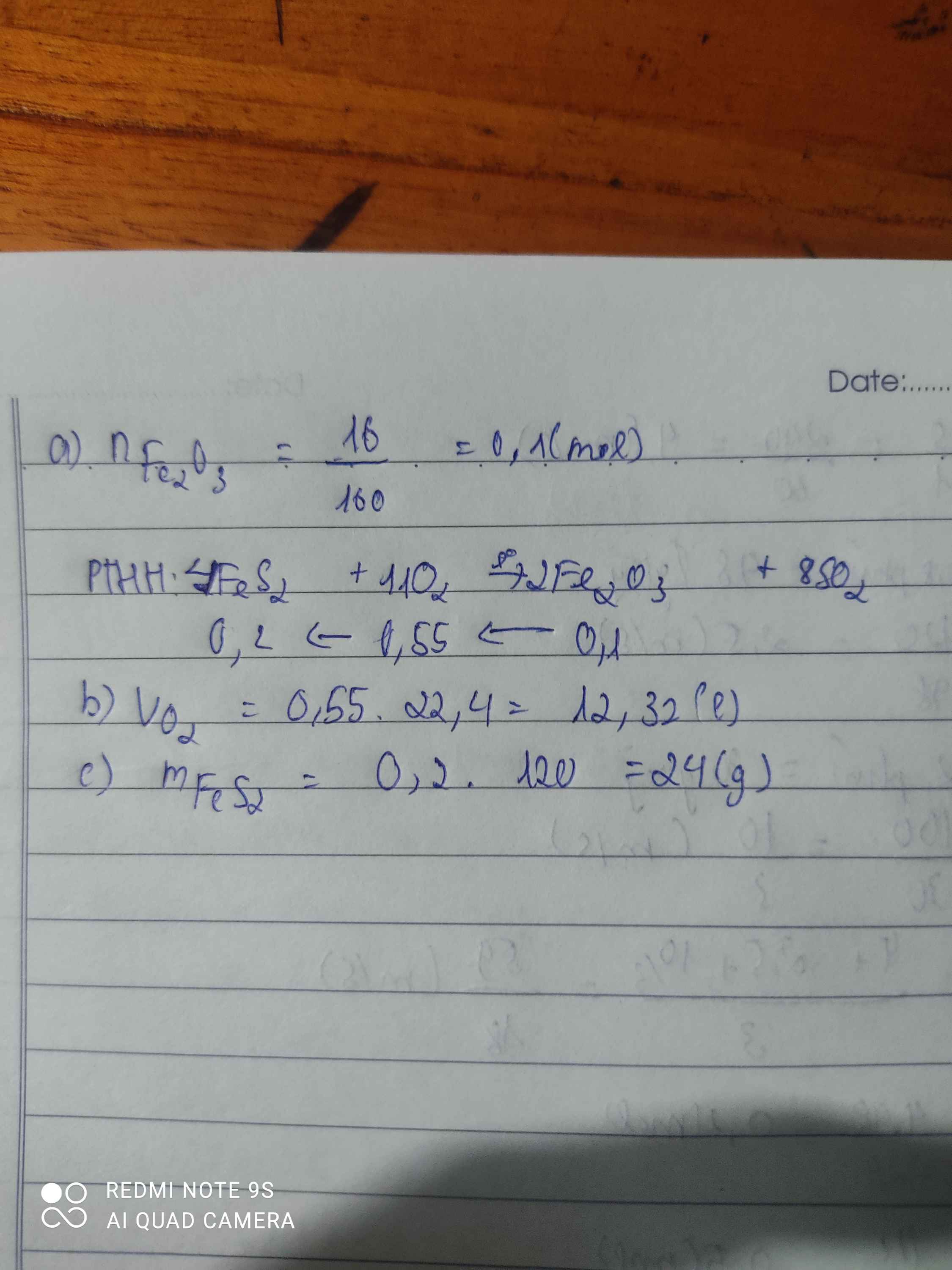
nFe=m/M=16,8/0,3(mol)
pt1: 4Fe +3O2 -t0-> 2Fe2O3
vậy: 0,3---------------->0,15(mol)
pt2: Fe2O3 + 3H2SO4 -> Fe2(SO4)3 + 3H2O
vậy: 0,15-------->0,45(mol)
=> mH2SO4=n.M=0,45.98=44,1(g)
Vậy m=44,1(g)
cứ theo cái đề bài mà làm ra thôi mà