Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(FeO+2HCl\rightarrow FeCl_2+H_2O\)
Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)=n_{Fe}\) \(\Rightarrow n_{FeO}=\dfrac{12,8-0,1\cdot56}{72}=0,1\left(mol\right)\)
Theo các PTHH: \(\Sigma n_{HCl}=2n_{Fe}+2n_{FeO}=0,4\left(mol\right)\)
\(\Rightarrow V_{HCl}=\dfrac{0,4}{0,1}=4\left(l\right)\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
Fe + 2HCl => FeCl2 + H2
0,1 0,2 0,1
=> FeO = \(\dfrac{12,8-0,1.56}{72}=0,1\left(mol\right)\)
FeO + 2HCl => FeCl2 + H2O
0,1 0,2
VHCl = 0,2 . 22,4 = 4,48 lít

a) 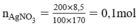
Phương trình hóa học của phản ứng:
HCl + AgNO3 → AgCl + HNO3
Theo pt nHCl = nAgCl = 0,1 mol
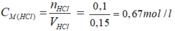
b) 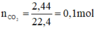
Phương trình hóa học của phản ứng:
HCl + NaHCO3 → NaCl + CO2↑ + H2O
Theo pt: nHCl = nCO2 = 0,1 mol ⇒ mHCl = 0,1. 36,5 = 3,65 g
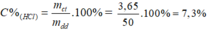

Bài 6 :
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
x ___________x _______x
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
y ____________y_______ y
\(n_{H2}=\frac{5,6}{22,4}=0,25\left(mol\right)\)
\(\rightarrow x+y=0,25\left(1\right)\)
\(2NaOH+FeCl_2\rightarrow Fe\left(OH\right)_2+2NaCl\)
__________ x ______ x
\(2NaOH+MgCl_2\rightarrow Mg\left(OH\right)_2+2NaCl\)
__________y _________ y
Ta có mFe(OH)2+mMg(OH)2=17,7
\(\rightarrow90x+58y=17,1\left(2\right)\)
(1)(2)\(\rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,15\end{matrix}\right.\)
\(\rightarrow m_{Fe}=0,1.56=5,6\left(g\right);m_{Al}=0,15.27=4,05\left(g\right)\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
0,1____0,15
\(Mg+Cl_2\rightarrow MgCl_2\)
0,15__0,15
\(V_{Cl2}=0,3.22,4=6,72\left(l\right)\)
Bài 2 :
\(n_{HBr}=\frac{1}{81},n_{NaOH}=\frac{1}{40}\)
\(n_{NaOH}>n_{HBr}\rightarrow\) Chuyển xanh
Bài 3 :
\(m\downarrow=m_{AgCl}=0,1.143,5=14,35\left(g\right)\)
Bài 4 :
\(m_{HX}=29,2\left(g\right)\rightarrow n_{HX}=\frac{29,2}{X+1}\left(mol\right)\)
\(n_{NaOH}=0,8\left(mol\right)=n_{HX}\)
\(\rightarrow\frac{29,2}{X+1}=0,8\Leftrightarrow X=35,5\left(Cl\right)\)
Vậy HX là HCl
Bài 5 :
a, \(Fe+2HCl\rightarrow FeCl_2+H_2\)
__a____________a________a
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
b_____________b_________b
\(\rightarrow a+b=0,25\left(1\right)\)
\(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_2\downarrow+2NaCl\)
a_________________a____________________
\(MgCl_2+2NaOH\rightarrow Mg\left(OH\right)_2+2NaCl\)
b___________________b_________________
\(m_{kettua}=17,7\left(g\right)\rightarrow90a+58b=17,7\left(2\right)\)
(1);(2) \(\rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,15\end{matrix}\right.\)
\(n_{Fe}=0,1\left(mol\right)\rightarrow m_{Fe}=0,1.56=5,6\left(g\right)\)
\(n_{Mg}=0,15\left(mol\right)\rightarrow m_{Mg}=0,15.24=3,6\left(g\right)\)
b, \(2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\)
0,1_____0,15_____________
\(Mg+Cl_2\underrightarrow{^{to}}MgCl_2\)
0,1___0,1__________
\(\rightarrow n_{Cl2}=0,15+0,1=0,25\left(mol\right)\)
\(\rightarrow V_{Cl2}=0,25.22,4=5,6\left(l\right)\)

PTHH: \(2KMnO_4+16HCl_{\left(đ\right)}\rightarrow2KCl+2MnCl_2+5Cl_2\uparrow+8H_2O\)
Ta có: \(n_{KMnO_4}=\dfrac{14,2}{158}=\dfrac{71}{790}\left(mol\right)\)
\(\Rightarrow n_{Cl_2}=\dfrac{71}{316}\left(mol\right)\) \(\Rightarrow V_{Cl_2}=\dfrac{71}{316}\cdot22,4\approx5,03\left(l\right)\)
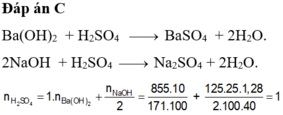
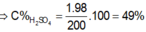
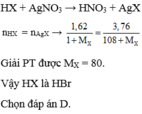
PTHH: \(HX+AgNO_3\rightarrow HNO_3+AgX\downarrow\)
Theo PTHH: \(n_{HX}=n_{AgX}\)
\(\Rightarrow\dfrac{40,5\cdot10\%}{M_X+1}=\dfrac{8,5}{108+M_X}\) \(\Rightarrow M_X\approx96,38\)
*Bạn xem lại đề