Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_S=\dfrac{3.2}{32}=0.1\left(mol\right)\)
\(n_{O_2}=\dfrac{1.12}{22.4}=0.05\left(mol\right)\)
\(S+O_2\underrightarrow{t^0}SO_2\)
\(0.05.0.05...0.05\)
\(\Rightarrow Sdư\)
\(V_{SO_2}=0.05\cdot22.4=1.12\left(l\right)\)
\(b.\)
\(S+O_2\underrightarrow{t^0}SO_2\)
\(0.1..0.1\)
\(V_{kk}=5V_{O_2}=5\cdot0.1\cdot22.4=11.2\left(l\right)\)

a, PT: \(S+O_2\underrightarrow{t^o}SO_2\)
Ta có: \(n_S=\dfrac{3,2}{32}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,05}{1}\), ta được S dư.
Theo PT: \(n_{SO_2}=n_{O_2}=0,05\left(mol\right)\) \(\Rightarrow V_{SO_2}=0,05.22,4=1,12\left(l\right)\)
b, Theo PT: \(n_{O_2}=n_S=0,1\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,1.22,4=2,24\left(l\right)\)
\(\Rightarrow V_{kk}=2,24.5=11,2\left(l\right)\)

Ta có: \(n_S=\dfrac{6,4}{32}=0,2\left(mol\right)\)
a. PTHH: S + O2 ---to---> SO2
Theo PT: \(n_{SO_2}=n_S=0,2\left(mol\right)\)
=> \(m_{SO_2}=0,2.64=12,8\left(g\right)\)
b. Theo PT: \(n_{O_2}=n_S=0,2\left(mol\right)\)
=> \(m_{O_2}=0,2.32=6,4\left(g\right)\)
a)S+O2-------->SO2
b)n S=6,4/32=0,2(mol)
Theo pthh
n SO2 =n S=0,2(mol)
V SO2=0,2.22,4=4,48(mol)

Gọi nC = a (mol); nS = b (mol)
12a + 32b = 12 (1)
PTHH:
C + O2 -> (t°) CO2
a ---> a ---> a
S + O2 -> (t°) SO2
b ---> b ---> b
44a + 64b = 28 (2)
Từ (1)(2) => a = 0,2 (mol); b = 0,3 (mol)
nO2 = 0,2 + 0,3 = 0,5 (mol)
VO2 = 0,5 . 22,4 = 11,2 (l)

S+O2-to>SO2
0,2--0,2----0,2 mol
n SO2=\(\dfrac{4,48}{22,4}\)=0,2 mol
=>m S=0,2.32=6,4g
=>VO2=0,2.22,4=4,48l

a) PTHH : \(S+O_2->SO_2\)
b) Ta có : \(n_S\) = \(\dfrac{m_S}{M_S}\) = 0.1 (mol)
Có : \(n_S=n_{O_2}\)
--> \(n_{O_2}\) = 0.1 (mol)
=> \(V_{O_2\left(đktc\right)}\) = \(n_{O_2}\) . 22.4 = 2.24 (L)
\(n_S=\dfrac{3,2}{32}=0,1\left(mol\right)\\ PTHH:S+O_2\underrightarrow{t^o}SO_2\\ \left(mol\right)..0,1\rightarrow0,1..0,1\\ V_{O_2}=0,1.22,4=2,24\left(l\right)\)

a) \(n_S=\dfrac{16}{32}=0,5\left(mol\right)\)
PTHH: S + O2 --to--> SO2
0,5->0,5------>0,5
=> mSO2 = 0,5.64 = 32 (g)
b) VO2 = 0,5.22,4 = 11,2 (l)
=> Vkk = 11,2.5 = 56 (l)
c)
\(n_{O_2}=\dfrac{24}{32}=0,75\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,5}{1}< \dfrac{0,75}{1}\)
=> S hết, O2 dư
PTHH: S + O2 --to--> SO2
0,5->0,5------>0,5
=> nO2(dư) = 0,75 - 0,5 = 0,25 (mol)

a) S + O2 --to--> SO2
b) \(n_{SO_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH: S + O2 --to--> SO2
0,5<-0,5<----0,5
=> \(V_{O_2}=0,5.22,4=11,2\left(l\right)\)
c) \(m_S=0,5.32=16\left(g\right)\)
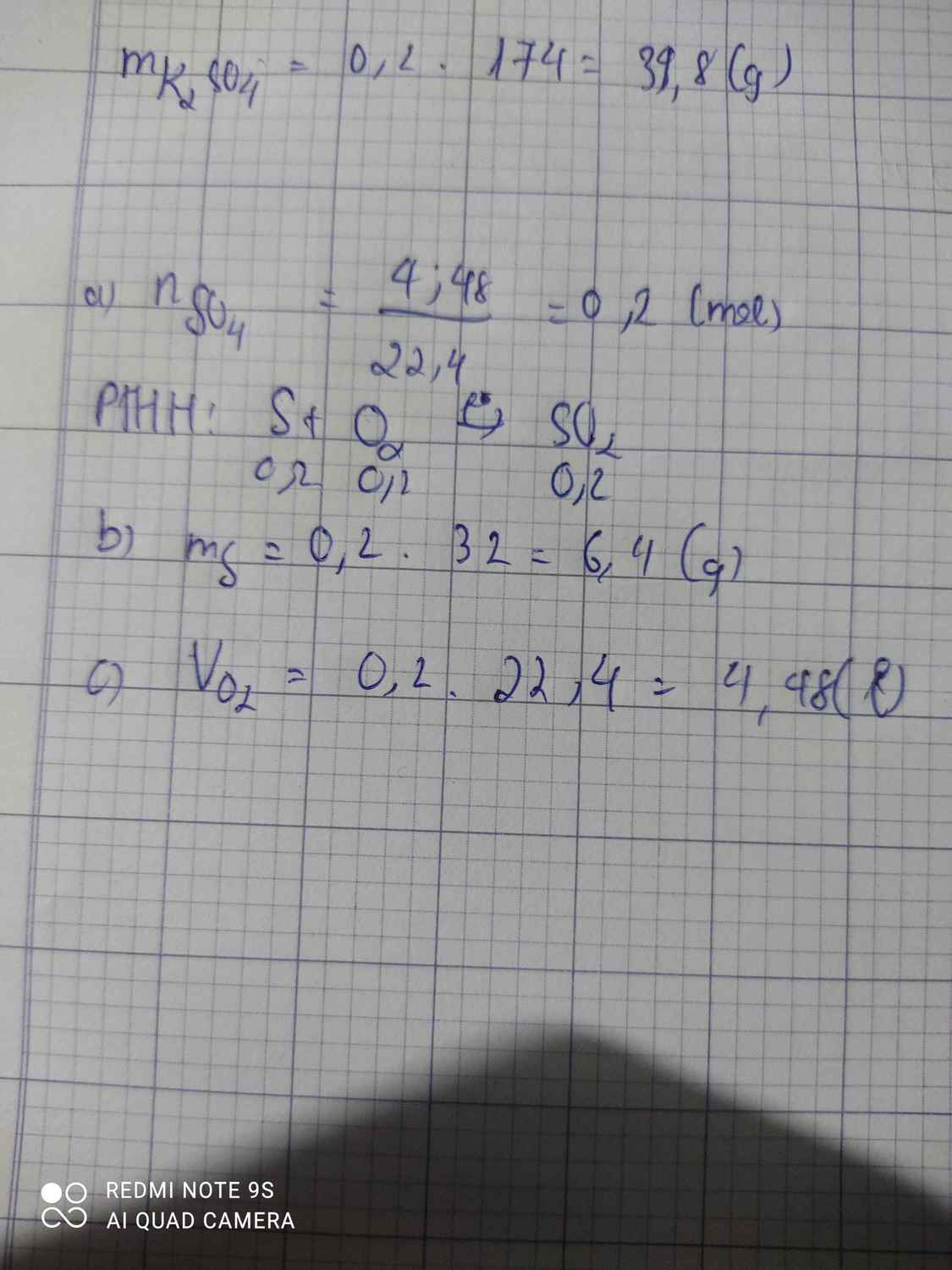
\(n_{O2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(S+O_2\underrightarrow{t^o}SO_2|\)
1 1 1
0,15 0,15 0,15
a) \(n_S=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_S=0,15.32=4,8\left(g\right)\)
b) \(n_{SO2}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
\(V_{SO2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
Chúc bạn học tốt