Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Fe}=\dfrac{5.6}{56}=0.1\left(mol\right)\)
\(Fe_2O_3+3H_2\underrightarrow{^{^{t^0}}}2Fe+3H_2O\)
\(0.05........0.15......0.1\)
\(\%Fe_2O_{3\left(bk\right)}=\dfrac{0.05\cdot160}{20}\cdot100\%=40\%0\%\)
\(V_{H_2}=0.15\cdot22.4=3.36\left(l\right)\)
\(m_X=20-0.05\cdot160+5.6=17.6\left(g\right)\)

PTHH: \(Fe_xO_y+yH_2\underrightarrow{t^o}xFe+yH_2O\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
Đặt \(\left\{{}\begin{matrix}n_{Fe\left(oxit\right)}=a\left(mol\right)=n_{H_2}\\n_{O\left(oxit\right)}=b\left(mol\right)\end{matrix}\right.\)
Ta có: \(m_{tăng}=m_{Fe}-m_{H_2}\) \(\Rightarrow56a-2a=3,24\) \(\Rightarrow a=n_{Fe}=0,06\left(mol\right)\)
Hỗn hợp D gồm \(\left\{{}\begin{matrix}n_{CO_2\left(dư\right)}=c\left(mol\right)\\n_{H_2O}=n_{O\left(oxit\right)}=b\left(mol\right)\end{matrix}\right.\)
Ta có hệ phương trình: \(\left\{{}\begin{matrix}c+b=0,1\\18b+2c=7,4\cdot2\cdot\left(b+c\right)\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}b=0,08\\c=0,02\end{matrix}\right.\)
\(\Rightarrow x:y=a:b=0,06:0,08=3:4\)
\(\Rightarrow\) Công thức cần tìm là Fe3O4

\(a)Fe_3O_4 + 4H_2 \xrightarrow{t^o} 3Fe + 4H_2O\\ b)n_{H_2} = \dfrac{8,96}{22,4} = 0,4(mol)\\ n_{Fe} = \dfrac{3}{4}n_{H_2} = 0,3(mol)\\ n_{Fe_3O_4\ pư} = \dfrac{1}{4}n_{H_2} = 0,1(mol)\\ \Rightarrow m_{chất\ rắn\ sau\ phản\ ứng} = 0,3.56 + (34,8 -0,1.232)=28,4(gam)\\ c) \%m_{Fe_3O_4\ bị\ khử} = \dfrac{0,1.232}{34,8}.100\% = 66,67\%\)

\(n_{H_2}=\dfrac{8.96}{22.4}=0.4\left(mol\right)\)
\(BTKL:\)
\(m+0.4\cdot2=28.4+7.2\)
\(\Rightarrow m=34.8\left(g\right)\)
\(b.\)
\(m_{Fe}=0.59155\cdot28.4=16.8\left(g\right)\)
\(n_{Fe}=\dfrac{16.8}{56}=0.3\left(mol\right)\)
\(PTHH:\)
\(\dfrac{x}{y}=\dfrac{n_{Fe}}{n_{H_2}}=\dfrac{0.3}{0.4}=\dfrac{3}{4}\)
\(CT:Fe_3O_4\)

a. \(PTHH:3H_2+Fe_2O_3\rightarrow2Fe+3H_2O\)
b. \(n_{Fe_2O_3}=\dfrac{m_{Fe_2O_3}}{M_{Fe_2O_3}}=\dfrac{40}{160}=0,25\left(mol\right)\)
- Mol theo PTHH : \(3:1:2:3\)
- Mol theo phản ứng : \(0,75\leftarrow0,25\rightarrow0,5\rightarrow0,75\)
\(\Rightarrow n_{Fe}=0,5\left(mol\right)\)
\(\Rightarrow m_{Fe}=n_{Fe}.M_{Fe}=0,5.56=28\left(g\right)\)
c. Ta có : \(n_{Fe_2O_3}=0,25\left(mol\right);n_{H_2}=0,3\left(mol\right)\)
Do \(0,25< 0,3\) ⇒ H2 dư.

a,\(n_{Mg}=\dfrac{9,6}{24}=0,4\left(mol\right);n_{H_2SO_4}=1,5.0,2=0,3\left(mol\right)\)
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: 0,3 0,3 0,3
Ta có: \(\dfrac{0,4}{1}>\dfrac{0,3}{1}\) ⇒ Mg dư, H2SO4 pứ hết
\(m_{MgSO_4}=0,3.120=36\left(g\right)\)
b,\(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
c, \(n_{Fe_2O_3}=\dfrac{6,4}{160}=0,04\left(mol\right)\)
PTHH: 3H2 + Fe2O3 → 2Fe + 3H2O
Mol: 0,04 0,08
Ta có: \(\dfrac{0,3}{3}>\dfrac{0,04}{1}\) ⇒ H2 dư, Fe2O3 pứ hết
\(\Rightarrow m_{Fe}=0,08.56=4,48\left(g\right)\)

\(n_{H_2}=\dfrac{4,032}{22,4}=0,18\left(mol\right)\)
PTHH: Fe + H2SO4 ---> FeSO4 + H2
0,18 <------------------------ 0,18
\(\rightarrow n_O=\dfrac{13,92-0,18.56}{16}=0,24\left(mol\right)\)
CTHH: FexOy
=> x : y = 0,18 : 0,24 = 3 : 4
CTHH Fe3O4
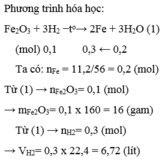
Fe2O3 + 3H2 \(\underrightarrow{to}\) 2Fe + 3H2O
\(n_{Fe}=\frac{11,2}{56}=0,2\left(mol\right)\)
a) Theo pT: \(n_{Fe_2O_3}pư=\frac{1}{3}n_{H_2}=\frac{1}{2}\times0,2=0,1\left(mol\right)\)
\(\Rightarrow m_{Fe_2O_3}=0,1\times160=16\left(g\right)\)
b) Theo PT: \(n_{H_2}pư=\frac{3}{2}n_{Fe}=\frac{3}{2}\times0,2=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}pư=0,3\times22,4=6,72\left(l\right)\)
c) \(m_{Fe_2O_3}dư=40-16=24\left(g\right)\)
\(m_X=m_{Fe_2O_3}dư+m_{Fe}=24+11,2=35,2\left(g\right)\)
nFe = 11,2 / 56 = 0,2 (mol)
Fe2O3 + 3H2 → 2Fe + 3H2O (t\(^o\))
mol pư: 0,1.←0,3 ←0,2
a, Vậy : m(Fe2O3 bị khử) = 0,1 * 160 = 16 (gam)
b, Vậy : V(H2 pư) = 0,3 * 22,4 = 6,72 (lít)
c, m(X) = m(Fe) + mFe2O3(dư)
= 11,2 + 40 - 16
= 35,2 (gam)