Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\); \(n_{A\left(OH\right)_2}=\dfrac{34,2}{M_A+34}\left(mol\right)\)
\(A+2H_2O\rightarrow A\left(OH\right)_2+H_2\)
\(\dfrac{34,2}{M_A+34}\) --> \(\dfrac{34,2}{M_A+34}\) ( mol )
\(\rightarrow n_{H_2}=\dfrac{34,2}{M_A+34}=0,2\left(mol\right)\)
\(\Leftrightarrow34,2=0,2M_A+6,8\)
\(\Leftrightarrow0,2M_A=27,4\)
\(\Leftrightarrow M_A=137\) ( g/mol )
--> A là Bari ( Ba )
\(A+H_2O\rightarrow A\left(OH\right)_2+H_2\\ n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ TheoPT:n_{H_2}=n_{A\left(OH\right)_2}=0,2\left(mol\right)\\ \Rightarrow M_{A\left(OH\right)_2}=A+17.2=\dfrac{34,2}{0,2}=171\\ \Rightarrow A=137\left(Ba\right)\)

\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(A+2H_2O\rightarrow A\left(OH\right)_2+H_2\)
\(................0.15.....0.15\)
\(M_{A\left(OH\right)_2}=\dfrac{11.1}{0.15}=74\left(\dfrac{g}{mol}\right)\)
\(\Rightarrow A=40\)
\(\Rightarrow B\)

Câu 8:
Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Xét tỉ lệ: \(\dfrac{0,1}{2}< \dfrac{0,2}{1}\), ta được O2 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{O_2\left(pư\right)}=\dfrac{1}{2}n_{H_2}=0,05\left(mol\right)\\n_{H_2O}=n_{H_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{O_2\left(dư\right)}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2\left(dư\right)}=0,15.22,4=3,36\left(l\right)\)
\(m_{H_2O}=0,1.18=1,8\left(g\right)\)
Bạn tham khảo nhé!
Câu 9:
a, PT: \(2R+O_2\underrightarrow{t^o}2RO\)
Theo ĐLBT KL, có: mR + mO2 = mRO
⇒ mO2 = 4,8 (g)
\(\Rightarrow n_{O_2}=\dfrac{4,8}{32}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b, Theo PT: \(n_R=2n_{O_2}=0,3\left(mol\right)\)
\(\Rightarrow M_R=\dfrac{19,2}{0,3}=64\left(g/mol\right)\)
Vậy: M là đồng (Cu).
Câu 10:
Ta có: mBaCl2 = 200.15% = 30 (g)
a, m dd = 200 + 100 = 300 (g)
\(\Rightarrow C\%_{BaCl_2}=\dfrac{30}{300}.100\%=10\%\)
⇒ Nồng độ dung dịch giảm 5%
b, Ta có: \(C\%_{BaCl_2}=\dfrac{30}{150}.100\%=20\%\)
⇒ Nồng độ dung dịch tăng 5%.
Bạn tham khảo nhé!

a) \(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
PTHH: Mg + H2SO4 --> MgSO4 + H2
0,1---->0,1------->0,1---->0,1
=> \(m_{dd.H_2SO_4}=\dfrac{0,1.98}{4,9\%}=200\left(g\right)\)
b) mdd sau pư = 2,4 + 200 - 0,1.2 = 202,2 (g)
mMgSO4 = 0,1.120 = 12 (g)
\(C\%_{MgSO_4}=\dfrac{12}{202,2}.100\%=5,9\%\)
c)
\(n_{CuO}=\dfrac{20}{80}=0,25\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
Xét tỉ lệ: \(\dfrac{0,25}{1}>\dfrac{0,1}{1}\) => Hiệu suất tính theo H2
\(n_{Cu}=\dfrac{3,2}{64}=0,05\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
0,05<-----0,05
=> \(H=\dfrac{0,05}{0,1}.100\%=50\%\)
a, \(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
PTHH: Mg + H2SO4 ---> MgSO4 + H2
0,1--->0,1---------->0,1-------->0,1
\(m_{dd\left(H_2SO_4\right)}=\dfrac{0,1.98}{4,9\%}=200\left(g\right)\)
b, \(m_{dd\left(sau.pư\right)}=2,4+200-0,2.2=202,2\left(g\right)\)
\(\rightarrow C\%_{MgSO_4}=\dfrac{0,1.120}{202,2}.100\%=5,93\%\)
c, \(n_{CuO}=\dfrac{20}{80}=0,25\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
LTL: 0,25 > 0,1 => CuO dư
\(n_{Cu}=\dfrac{3,2}{64}=0,05\left(mol\right)\)
Theo pt: \(n_{H_2}=n_{Cu}=0,05\left(mol\right)\)
=> \(H=\dfrac{0,05}{0,1}.100\%=50\%\)
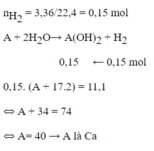

`A + 2H_2 O -> A(OH)_2 + H_2`
`n_[H_2] = [ 4,48 ] / [22,4 ] = 0,2 (mol)`
`n_[A(OH)_2] = [ 34,2 ] / [ M_A + 34 ] (mol)`
Mà `n_[H_2] = n_[A(OH)_2]`
`=> [34,2] / [M_A + 34] = 0,2`
`<=>M_A = 137 ( g // mol)`
`-> A` là `Ba`