Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Vì Cu không tác dụng với HCl loãng :
\(n_{H2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,1 0,1
\(n_{Fe}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(m_{Fe}=0,1.56=5,6\left(g\right)\)
\(m_{Cu}=9,5-5,6=3,9\left(g\right)\)
0/0Fe = \(\dfrac{5,6.100}{9,5}=58,95\)0/0
0/0Cu = \(\dfrac{3,9.100}{9,5}=41,05\)0/0
Chúc bạn học tốt

\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
a 2a a a
\(FeS+2HCl\rightarrow FeCl_2+H_2S\uparrow\)
b 2b b b
\(n_{HCl}=\dfrac{400\times7.3}{100\times36.5}=0.8mol\)
\(n_X=\dfrac{4.48}{22.4}=0.2mol\)
\(M_X=2\times9=18\Leftrightarrow\dfrac{2a+34b}{a+b}=18\)
Ta có: \(\left\{{}\begin{matrix}a+b=0.2\\\dfrac{2a+34b}{a+b}=18\end{matrix}\right.\)\(\Leftrightarrow\left\{{}\begin{matrix}a+b=0.2\\2a+34b=3.6\end{matrix}\right.\)\(\Leftrightarrow\left\{{}\begin{matrix}a=0.1\\b=0.1\end{matrix}\right.\)
a. \(\%V_{H_2}=\dfrac{0.1\times22.4\times100}{4.48}=50\%\)
\(\%V_{H_2S}=100-50=50\%\)
b. \(a=0.1\times56+0.1\times88=14.4g\)
\(\%m_{Fe}=\dfrac{0.1\times56}{14.4}\times100=38.8\%\)
\(\%m_{FeS}=100-38.8=61.2\%\)
c. m dung dịch sau phản ứng\(=14.4+400-0.1\times2-0.1\times34=410.8g\)
nHCl phản ứng\(=2\times0.1+2\times0.1=0.4mol\)
nHCl dư = 0.8 - 0.4 = 0.4 mol
\(C\%_{HCldu}=\dfrac{0.4\times36.5\times100}{410.8}=3.55\%\)
\(C\%_{FeCl_2}=\dfrac{0.2\times127\times100}{410.8}=6.18\%\)

PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)=n_{Fe}\)
\(\Rightarrow\left\{{}\begin{matrix}n_{HCl}=0,2\left(mol\right)\\\%m_{Fe}=\dfrac{0,1\cdot56}{12}\cdot100\%\approx46,67\%\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{HCl}}=\dfrac{0,2}{0,2}=1\left(M\right)\\\%m_{Cu}=53,33\%\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Cu không phản ứng
\(nH_2=nFe=\dfrac{2,24}{22,4}=0,1mol\)
\(\rightarrow mFe=0,1.56=5,6gam\)
\(\rightarrow\%mFe=\dfrac{5,6}{12}.100\%=46,\left(6\right)\%\)
\(\rightarrow\%mCu=100\%-46,\left(6\right)\%=53,\left(3\right)\%\)
c)
\(CM_{HCl}=\dfrac{0,1.2}{0,2}=1M\)

\(n_{H_2}=\dfrac{1,12}{22,4}=0,05(mol)\\ Fe+2HCl\to FeCl_2+H_2\\ \Rightarrow n_{Fe}=0,05(mol)\\ \Rightarrow \%_{Fe}=\dfrac{0,05.56}{20}.100\%=14\%\\ \Rightarrow \%_{Cu}=100\%-14\%=86\%\)

\(n_{Mg}=x(mol);n_{Fe}=y(mol)\\ \Rightarrow 24x+56y=4(1)\\ n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)\\ Mg+2HCl\to MgCl_2+H_2\\ Fe+2HCl\to FeCl_2+H_2\\ \Rightarrow x+y=0,1(2)\\ (1)(2)\Rightarrow x=y=0,05(mol)\\ \Rightarrow \%_{Fe}=\dfrac{0,05.56}{4}.100\%=70\%\\ \Rightarrow \%_{Mg}=100\%-70\%=30\%\)

a)
TN1: Gọi (nZn; nFe; nCu) = (a; b; c)
=> 65a + 56b + 64c = 18,5 (1)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
a---------------------->a
Fe + 2HCl --> FeCl2 + H2
b----------------------->b
=> a + b = 0,2 (2)
TN2: Gọi (nZn; nFe; nCu) = (ak; bk; ck)
=> ak + bk + ck = 0,15 (3)
PTHH: Zn + Cl2 --to--> ZnCl2
ak-->ak
2Fe + 3Cl2 --to--> 2FeCl3
bk--->1,5bk
Cu + Cl2 --to--> CuCl2
ck-->ck
=> \(ak+1,5bk+ck=\dfrac{3,92}{22,4}=0,175\)(4)
(1)(2)(3)(4) => \(\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,1\left(mol\right)\\c=0,1\left(mol\right)\\k=0,5\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{0,1.65}{18,5}.100\%=35,135\%\\\%m_{Fe}=\dfrac{0,1.56}{18,5}.100\%=30,27\%\\\%m_{Cu}=\dfrac{0,1.64}{18,5}.100\%=34,595\%\end{matrix}\right.\)
b) nO(oxit) = \(\dfrac{23,7-18,5}{16}=0,325\left(mol\right)\)
=> nH2O = 0,325 (mol)
=> nHCl = 0,65 (mol)
=> \(V=\dfrac{0,65}{1}=0,65\left(l\right)=650\left(ml\right)\)
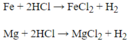
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(FeS+2HCl\rightarrow FeCl_2+H_2S\uparrow\)
Đặt nH2 = x mol; nH2S = y mol
Ta có: nkhí = x + y = 0,1 mol;
mkhí = 2x + 34y = 0,1.9.2 = 1,8 gam
Giải hệ ta có: x = 0,05 và y = 0,05
Suy ra nFe = 0,05. nFeS = 0,05 mol.
Vậy %nFe = 50%.
\(n_{\uparrow}=\dfrac{2,24}{22,4}=0,1mol\)
Gọi \(\left\{{}\begin{matrix}n_{Fe}=x\left(mol\right)\\n_{FeS}=y\left(mol\right)\end{matrix}\right.\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(FeS+2HCl\rightarrow FeCl_2+H_2S\uparrow\)
\(\Rightarrow x+y=0,1\left(1\right)\)
\(d_{\uparrow}\)/H2=9\(\Rightarrow\overline{M_{\uparrow}}=9\cdot2=18\)
Sơ đồ chéo:
Fe 56 70
18
FeS 88 38
\(\Rightarrow\dfrac{n_{Fe}}{n_{FeS}}=\dfrac{70}{38}=\dfrac{35}{19}=\dfrac{x}{y}\)(2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=\dfrac{7}{108}\\y=\dfrac{19}{540}\end{matrix}\right.\)
\(\%Fe=\dfrac{\dfrac{7}{108}}{\dfrac{7}{108}+\dfrac{19}{540}}\cdot100\%=64,81\%\)