
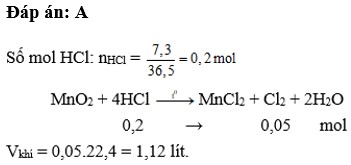
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

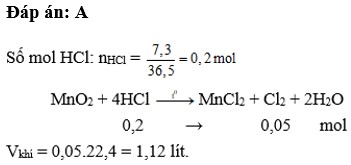

\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
\(n_{HCl}=0,2.1=0,2\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,2}{2}\) => HCl hết, Fe dư
PTHH: Fe + 2HCl --> FeCl2 + H2
__________0,2---------------->0,1
=> VH2 = 0,1.22,4 = 2,24(l)
=> B

1. B
2. B
(Câu 2 cậu nên sửa lại câu hỏi nhé: Khối lượng dung dịch NaOH 10% ...)
Câu 1.
\(n_{Fe}=\dfrac{5,6}{56}=0,1mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,1 0,1
\(V_{H_2}=0,1\cdot22,4=2,24\left(l\right)\)
Chọn B.
Câu 2. \(n_{HCl}=0,2\cdot1=0,2mol\)
Để trung hòa: \(\Rightarrow n_{H^+}=n_{OH^-}=0,2\)
\(m_{NaOH}=0,2\cdot40=8\left(g\right)\)
\(m_{ddNaOH}=\dfrac{8}{10\%}\cdot100\%=80\left(g\right)\)
Chọn B.

$CaCO_3 + 2HCl \to CaCl_2 + CO_2 + H_2O$
$n_{CO_2} = n_{CaCO_3} = \dfrac{50}{100} = 0,5(mol)$
$V_{CO_2} = 0,5.22,4 = 11,2(lít)$
Đáp án A

Đặt x,y, z lần lượt là số mol của Na,Al,Mg trong m gam hỗn hợp A
m gam A + H2O dư
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
x--------------------x--------->0,5x
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
x<------x-------------------------------------->1,5x
=> \(0,5x+1,5x=\dfrac{2,24}{22,4}=0,1\left(mol\right)\) (1)
2m gam A + NaOH
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
2x------------------------------->x
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
2y---------------------------------------------->3y
=> \(x+3y=\dfrac{8,96}{22,4}=0,4\left(mol\right)\) (2)
3m gam A + HCl
\(Na+HCl\rightarrow NaCl+\dfrac{1}{2}H_2\)
3x--------------------------->1,5x
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
3y----------------------------->4,5y
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
3z----------------------------->3z
=> \(1,5x+4,5y+3z=\dfrac{22,4}{22,4}=1\left(mol\right)\) (3)
Từ (1), (2), (3) =>\(\left\{{}\begin{matrix}x=0,05\\y=\dfrac{7}{60}\\z=\dfrac{2}{15}\end{matrix}\right.\)
=> \(m_{Na}=0,05.23=1,15\left(g\right)\)
\(m_{Al}=\dfrac{7}{60}.27=3,15\left(g\right)\)
\(m_{Mg}=\dfrac{2}{15}.24=3,2\left(g\right)\)
=> \(m=1,15+3,15+3,2=7,5\left(g\right)\)
=> \(\%m_{Na}=\dfrac{1,15}{7,5}.100=15,33\%\)
\(\%m_{Al}=\dfrac{3,15}{7,5}.100=42\%\)
\(\%m_{Mg}=\dfrac{3,2}{7,5}.100=42,67\%\)

\(2Na+2H2O\rightarrow2NaOH+H2\left(1\right)\)
\(2Al+2NaOH+2H2O\rightarrow2NaAlO2+3H2\left(2\right)\)
\(2Al+6HCl\rightarrow2AlCl3+3H2\left(3\right)\)
\(2Na+2HCl\rightarrow2NaCl+H2\left(4\right)\)
\(Mg+2HCl\rightarrow MgCl2+H2\left(5\right)\)
\(n_{H2\left(1\right)}=0,1\left(mol\right)\rightarrow n_{Na}=0,2\left(mol\right)\rightarrow m_{Na}=4,6\left(g\right)\)
\(n_{H2\left(2\right)}=0,4\left(mol\right)\Rightarrow n_{Al}=\dfrac{4}{15}\left(mol\right)\Rightarrow m_{Al}=7,2\left(g\right)\)
\(\Rightarrow n_{H2\left(3\right)}=\dfrac{3}{2}n_{Al}=0,4\left(mol\right)\)
\(n_{H2\left(4\right)}=\dfrac{1}{2}n_{Na}=0,1\left(mol\right)\)
\(\Rightarrow n_{H2\left(5\right)}=1-0,4-0,1=0,5\left(mol\right)\)
\(\Rightarrow n_{Mg}=0,5\left(mol\right)\Rightarrow m_{Mg}=12\left(g\right)\)
\(\Rightarrow m=12+4,6+7,2=23,8\left(g\right)\)
\(\%m_{Na}=\dfrac{4,6}{23,8}.100\%=19,33\%\)
\(\%m_{Al}=\dfrac{7,2}{23,8}.100\%=30,25\%\)
\(\%m_{Mg}=100-19,33-30,25=50,42\%\)
Chúc bạn học tốt