Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ KL:M\\ M+2HCl\rightarrow MCl_2+H_2\\ n_{MCl_2}=n_M=n_{H_2}=0,05\left(mol\right)\\ M_{MCl_2}=\dfrac{4,75}{0,05}=95\left(\dfrac{g}{mol}\right)\\ M\text{à}:M_{MCl_2}=M_M+71\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_M+71=95\\ \Leftrightarrow M_M=24\left(\dfrac{g}{mol}\right)\\ \Rightarrow M\left(II\right):Magie\left(Mg=24\right)\\ a=24.0,05=1,2\left(g\right)\)

\(a,2KMnO_4+16HCl_{đặc}\rightarrow\left(t^o\right)2KCl+2MnCl_2+5Cl_2+8H_2O\\ 2Fe+3Cl_2\rightarrow\left(t^o\right)2FeCl_3\\ Ta.có:n_{FeCl_3}=\dfrac{39}{162,5}=0,24\left(mol\right)\\ n_{Fe}=n_{FeCl_3}=0,24\left(mol\right)\\ n_{Cl_2}=\dfrac{3}{2}.0,24=0,36\left(mol\right)\\ n_{K_2MnO_4}=\dfrac{2}{5}.0,36=0,144\left(mol\right)\\ n_{HCl}=\dfrac{16}{5}.0.36=1,152\left(mol\right)\\ \Rightarrow a=m_{KMnO_4}=0,144.158=22,752\left(g\right)\\ b=C_{MddHCl}=\dfrac{1,152}{0,1}=11,52\left(M\right)\\ x=m_{Fe}=0,24.56=13,44\left(g\right)\\ V=V_{Cl_2\left(đktc\right)}=0,36.22,4=8,064\left(l\right) \)
\(b,n_{KCl}=n_{MnCl_2}=\dfrac{2}{5}.0,36=0,144\left(mol\right)\\ KCl+AgNO_3\rightarrow AgCl\downarrow\left(trắng\right)+KNO_3\\ MnCl_2+2AgNO_3\rightarrow2AgCl\downarrow\left(trắng\right)+Mn\left(NO_3\right)_2\\ n_{AgNO_3}=n_{AgCl}=n_{KCl}+2.n_{MnCl_2}=0,144+2.0,144=0,432\left(mol\right)\\ \Rightarrow m_{AgCl\downarrow\left(trắng\right)}=143,5.0,432=61,992\left(g\right)\\ m_{AgNO_3}=0,432.170=73,44\left(g\right)\\ \Rightarrow m_{ddAgNO_3}=\dfrac{73,44.100}{5}=1468,8\left(g\right)\)

nMg = 0,1(mol)
PTHH: Mg + 2HCl --> MgCl2 +H2
nMg = nMgCl2= nH2 = 0,1(mol)
=> mmuối = 9,5(g)
VH2 = 2,24(l)
b) CMHCl = 0,2/0,1=2(M)

\(\text{Đ}\text{ặt}:A\\ A+HCl\rightarrow ACl+H_2\\ n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ n_A=n_{ACl_2}=2.n_{H_2}=0,1.2=0,2\left(mol\right)\\ M_{ACl}=\dfrac{11,7}{0,2}=58,5\left(\dfrac{g}{mol}\right)\\ M\text{à}:M_{ACl}=M_A+35,5\\ \Rightarrow M_A=23\left(\dfrac{g}{mol}\right)\\ \Rightarrow A:Natri\left(Na\right)\\ a=23.0,2=4,6\left(g\right)\)
nH2=2,24/22,4=0,1(mol)
2M+2HCl→2MCl+H2
0,2 ← 0,2 ← 0,1
Có 0,2 .(M+35,5)=11,7(gam)
⇒ M=23 ⇒M là Na
mNa=23. 0,2= 4,6 (gam)

a) \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH: R + 2HCl --> RCl2 + H2
_____0,25<----------------0,25
=> \(\dfrac{34,25}{0,25}=137\left(Ba\right)\)
b) Muối thu được là BaCl2
Hiệu độ âm điện = 3,16 - 0,89 = 2,27
=> Liên kết ion

\(n_{Na}=\dfrac{2.3}{23}=0.1\left(mol\right)\)
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
\(0.1.....................0.1...........0.05\)
\(V_{H_2}=0.05\cdot22.4=1.12\left(l\right)\)
\(n_{CuSO_4}=\dfrac{160\cdot10}{100\cdot160}=0.1\left(mol\right)\)
Em xem lại đề vì kết tủa chỉ có duy nhất là : CuSO4 nhé.
\(CuSO_4+2NaOH\rightarrow Cu\left(OH\right)_2+Na_2SO_4\)
\(0.05..............0.1..............0.05\)
\(m_{Cu\left(OH\right)_2}=0.05\cdot98=4.9\left(g\right)\)

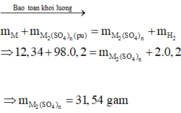
Gọi KL hoá trị II cần tìm là B
\(a,B+Br_2\rightarrow BBr_2\\ a=m_B=m_{BBr_2}-m_{Br_2}=22,08-\dfrac{2,688}{22,4}.160=2,88\left(g\right)\\ n_B=n_{BBr_2}=n_{Br_2}=\dfrac{2,688}{22,4}=0,12\left(mol\right)\\ \Rightarrow M_B=\dfrac{2,88}{0,12}=24\left(\dfrac{g}{mol}\right)\\ \Rightarrow B\left(II\right):Magie\left(Mg=24\right)\\ b,MgBr_2+Na_2CO_3\rightarrow MgCO_3\downarrow+2NaBr\\ n_{Na_2CO_3}=0,1.1=10,1\left(mol\right)\\ Vì:\dfrac{0,12}{1}>\dfrac{0,1}{1}\Rightarrow MgBr_2dư\\ \Rightarrow n_{MgCO_3}=n_{Na_2CO_3}=0,1\left(mol\right)\\ \Rightarrow b=m_{MgCO_3}=84.0,1=8,4\left(g\right)\)