Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,2 0,1
\(NaO+H_2O\rightarrow2NaOH\)
b)\(m_{Na}=0,2\cdot23=4,6g\)
\(m_{NaO}=m_{hh}-m_{Na}=40,5-4,6=35,9g\)
c)\(n_{NaO}=\dfrac{35,9}{39}=0,92mol\Rightarrow n_{NaOH}=2n_{NaO}=1,84mol\)
\(\Rightarrow m_{NaOH}=1,84\cdot40=73,6g\)

a. PTHH:
\(Ca+2H_2O--->Ca\left(OH\right)_2+H_2\left(1\right)\)
\(CaO+H_2O--->Ca\left(OH\right)_2\left(2\right)\)
b. Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT(1): \(n_{Ca}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{Ca}=0,1.40=4\left(g\right)\)
\(\Rightarrow\%_{m_{Ca}}=\dfrac{4}{9,6}.100\%=41,7\%\)
\(\%_{m_{CaO}}=100\%-41,7\%=58,3\%\)
c. Ta có: \(n_{CaO}=\dfrac{9,6-4}{56}=0,1\left(mol\right)\)
Ta có: \(n_{hh}=0,1+0,1=0,2\left(mol\right)\)
Theo PT(1,2): \(n_{Ca\left(OH\right)_2}=n_{hh}=0,2\left(mol\right)\)
\(\Rightarrow m_{Ca\left(OH\right)_2}=0,2.74=14,8\left(g\right)\)

\(a,n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ PTHH:2K+2H_2O\rightarrow2KOH+H_2\uparrow\\ Theo.pt:n_K=2n_{H_2}=2.0,1=0,2\left(mol\right)\\ m_K=0,2.39=7,8\left(g\right)\\ m_{K_2O}=17,2-7,8=9,4\left(g\right)\\ b,n_{CuO\left(bđ\right)}=\dfrac{12}{80}=0,15\left(mol\right)\\ PTHH:CuO+H_2\underrightarrow{t^o}Cu+H_2O\\ LTL:0,15>0,1\Rightarrow Cu.dư\)
Gọi nCuO (pư) = a (mol)
=> nCu = a (mol)
mchất rắn sau pư = 80(0,15 - a) + 64a = 10,8
=> a = 0,075 (mol)
=> nH2 (pư) = 0,075 (mol)
\(H=\dfrac{0,075}{0,1}=75\%\)

a) \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH:
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\) (1)
\(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\) (2)
Theo PT (1): \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Al}=0,2.27=5,4\left(g\right)\\m_{Al_2O_3}=15,6-5,4=10,2\left(g\right)\end{matrix}\right.\)
b) \(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
Theo PT (1), (2): \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}+n_{Al_2O_3}=0,2\left(mol\right)\)
\(\Rightarrow m_{mu\text{ố}i}=m_{Al_2\left(SO_4\right)_3}=0,2.342=68,4\left(g\right)\)
c) Theo PT (1), (2): \(n_{H_2SO_4}=n_{H_2}+3n_{Al_2O_3}=0,6\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4\left(c\text{ần}.d\text{ùng}\right)}=0,6.98=58,8\left(g\right)\)

Đổi 2,016 dm3 = 2,016 l
nH2 = 2,016/22,4 = 0,09 (mol)
Gọi nFe2O3 = a (mol); nCuO = b (mol)
160a + 80b = 5,6 (g) (1)
PTHH:
Fe2O3 + 3H2 -> (t°) 2Fe + 3H2O
Mol: a ---> 3a ---> 2a ---> 3a
CuO + H2 -> (t°) Cu + H2O
Mol: b ---> b ---> b ---> b
3a + b = 0,09 (mol) (2)
Từ (1) và (2) => a = 0,02 (mol); b = 0,03 (mol)
mFe2O3 = 0,02 . 160 = 3,2 (g)
mCuO = 0,03 . 80 = 2,4 (g)
mH2O = (0,02 . 3 + 0,03) . 18 = 1,62 (g)
mFe = 2 . 0,02 . 56 = 2,24 (g)
mCu = 0,03 . 64 = 1,92 (g)

a) PTHH : \(FeO+H_2-t^o->Fe+H_2O\)
\(CuO+H_2-t^o->Cu+H_2O\)
Đặt \(\hept{\begin{cases}n_{FeO}=x\left(mol\right)\\n_{CuO}=y\left(mol\right)\end{cases}}\) => \(72x+80y=11,2\left(I\right)\)
Có : \(m_{O\left(lấy.đi\right)}=m_{giảm}=1,92\left(g\right)\)
=> \(n_{O\left(lấy.đi\right)}=\frac{1,92}{16}=0,12\left(mol\right)\) Vì H% = 80% => Thực tế : \(n_{O\left(hh\right)}=\frac{0,12}{80}\cdot100=0,15\left(mol\right)\)
BT Oxi : \(x+y=0,15\left(II\right)\)
Từ (I) và (II) suy ra : \(\hept{\begin{cases}x=0,1\\y=0,05\end{cases}}\)
=> \(\hept{\begin{cases}m_{FeO}=7,2\left(g\right)\\m_{CuO}=4\left(g\right)\end{cases}}\)
b) PTHH : \(Fe+H_2SO_4-->FeSO_4+H_2\)
BT Fe : \(n_{Fe}=n_{FeO}=0,1\left(mol\right)\)
Theo pthh : \(n_{H_2}=n_{Fe}=0,1\left(mol\right)\)
=> \(V_{H_2}=2,24\left(l\right)\)
BT Cu : \(n_{Cu}=n_{CuO}=0,05\left(mol\right)\)
=> \(m_{CR\left(ko.tan\right)}=0,05\cdot64=3,2\left(g\right)\)

a) 4,4 gam kim loại không tan là Cu
`=> m_{Cu} = 4,4 (g)`
`=> m_{Al} + m_{Mg} = 15,5 - 4,4 = 11,1 (g)`
Gọi \(\left\{{}\begin{matrix}n_{Al}=a\left(mol\right)\\n_{Mg}=b\left(mol\right)\end{matrix}\right.\left(a,b>0\right)\)
`=> 27a + 24b = 11,1 (1)`
\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH:
2Al + 6HCl ---> 2AlCl3 + 3H2
a-------------------------->1,5a
Mg + 2HCl ---> MgCl2 + H2
b-------------------------->b
`=> 1,5a + b = 0,5(2)`
Từ `(1), (2) => a = 0,1; b = 0,35`
b) Đặt CTTQ của oxit kim loại là \(M_xO_y\) (M có hóa trị 2y/x và M có hóa trị n khi phản ứng với HCl)
PTHH:
\(M_xO_y+yH_2\xrightarrow[]{t^o}xM+yH_2O\)
Theo PTHH: \(n_{O\left(\text{ox}it\right)}=n_{H_2}=0,5\left(mol\right)\)
`=>` \(m_M=m_{M_xO_y}-m_{O\left(\text{ox}it\right)}=24,25-0,5.16=16,25\left(g\right)\)
\(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
\(2M+2nHCl\rightarrow2MCl_n+nH_2\)
\(\dfrac{0,5}{n}\)<---------------------------0,25
`=>` \(M_M=\dfrac{16,25}{\dfrac{0,5}{n}}=32,5n\left(g/mol\right)\)
Chỉ có n = 2 thỏa mãn `=> M_M = 32,5.2 = 65 (g//mol)`
Vậy kim loại M là kẽm (Zn)
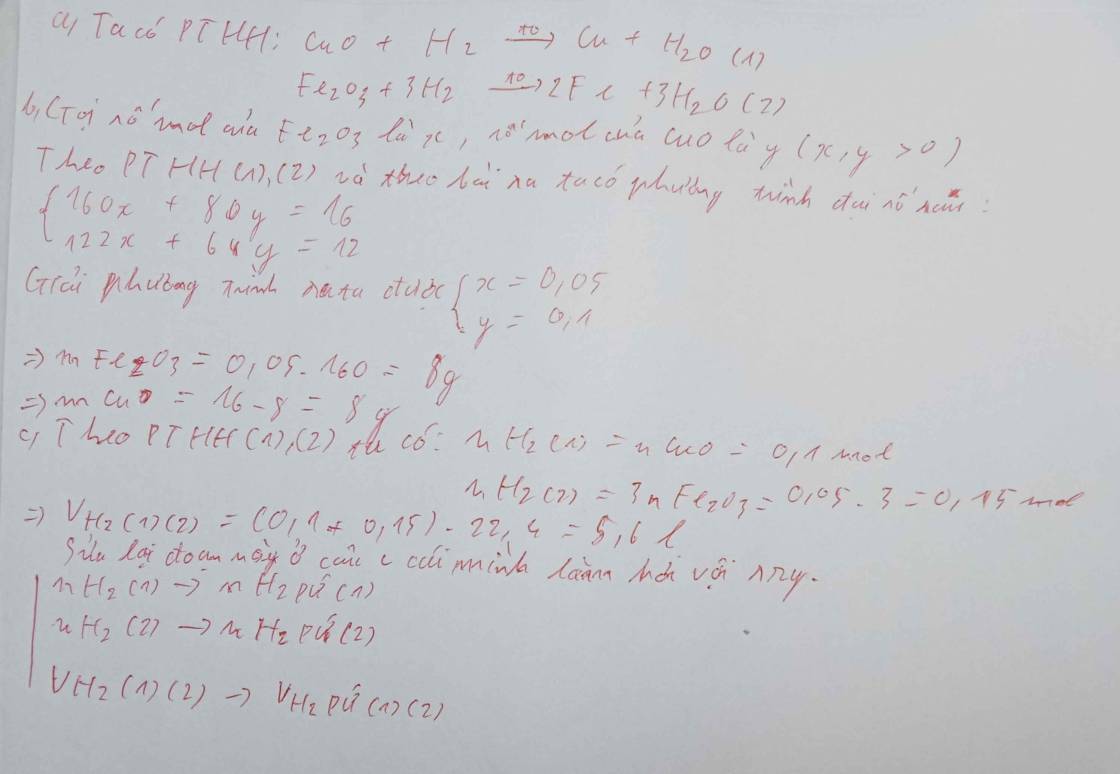
\(a.Ca+H_2O\rightarrow Ca\left(OH\right)_2+H_2\\ CaO+H_2O\rightarrow Ca\left(OH\right)_2\\ b.n_{H_2}=n_{Ca}=0,1\left(mol\right)\\ \Rightarrow m_{Ca}=0,1.40=4\left(g\right)\\ \Rightarrow m_{CaO}=9,6-4=5,6\left(g\right)\\ c.n_{CaO}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ \Sigma n_{Ca\left(OH\right)_2}=n_{Ca}+n_{CaO}=0,1+0,1=0,2\left(mol\right)\\ \Rightarrow m_{Ca\left(OH\right)_2}=0,2.74=14,8\left(g\right)\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ pthh:Ca+2H_2O\rightarrow Ca\left(OH\right)_2+H_2\)
0,1 0,1 0,1
\(CaO+H_2O\rightarrow Ca\left(OH\right)_2\)(2)
\(m_{Ca}=0,1.40=4\left(g\right)\\ m_{CaO}=9,6-4=5,6\left(g\right)\)
\(n_{CaO}=\dfrac{5,6}{56}=0,1\left(mol\right)\\ n_{Ca\left(OH\right)_2\left(2\right)}=n_{CaO}=0,1\left(mol\right)\)
\(n_{Ca\left(OH\right)_2}=\left(0,1+0,1\right).74=14,8\left(g\right)\)