Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
$C_2H_5OH + CH_3COOH \buildrel{{H_2SO_4,t^o}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O$
b)
n CH3COOC2H5 = n C2H5OH = 9,2/46 = 0,2(mol)
=> m este = 0,2.88 = 17,6 gam
c)
n este = 8,8/88 = 0,1(mol)
=> n C2H5OH = n CH3COOH = 0,1/60% = 1/6 mol
=> m C2H5OH = 46 . 1/6 = 7,67(gam) ; m CH3COOH = 60 . 1/6 = 10(gam)

Ta có: \(n_{Fe}=\dfrac{28}{56}=0,5\left(mol\right)\)
a, PT: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
______0,5____0,5_____0,5_____0,5 (mol)
b, mH2SO4 = 0,5.98 = 49 (g)
c, mFeSO4 = 0,5.152 = 76 (g)
d, \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
____0,5__0,5 (mol)
⇒ mCu = 0,5.64 = 32 (g)
Bạn tham khảo nhé!

nC2H5OH = 8.05/46 = 0.175 (mol)
nCH3COOH = 36/60 = 0.6 (mol)
nCH3COOC2H5 = 12.32/88 = 0.14 (mol)
C2H5OH + CH3COOH <-H2SO4đ,t0-> CH3COOC2H5 + H2O
1.......................1
0.175................0.6
LTL : 0.175/1 < 0.6/1
=> CH3COOH dư
mCH3COOH (dư) = ( 0.6 - 0.175) * 60 = 25.5 (g)
nCH3COOC2H5 = nC2H5OH = 0.175 (mol)
H% = 0.14/0.175 * 100% = 80%

\(n_{CH_3COOH}=\dfrac{6}{60}=0,1\left(mol\right)\)
\(n_{C_2H_5OH}=\dfrac{9,2}{46}=0,2\left(mol\right)\)
PT: \(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\) (xt, to)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,2}{1}\), ta được C2H5OH dư.
Theo PT: \(n_{CH_3COOC_2H_5}=n_{CH_3COOH}=0,1\left(mol\right)\)
\(\Rightarrow m_{CH_3COOC_2H_5}=0,1.88=8,8\left(g\right)\)

\(n_{CH_3COOC_2H_5}=\dfrac{4,4}{88}=0,05\left(mol\right)\)
PTHH: CH3COOH + C2H5OH --H2SO4(đ),to--> CH3COOC2H5 + H2O
0,05<--------------------------------------0,05
=> \(m_{CH_3COOH\left(lý.thuyết\right)}=0,05.60=3\left(g\right)\)
=> \(m_{CH_3COOH\left(tt\right)}=\dfrac{3.100}{60}=5\left(g\right)\)
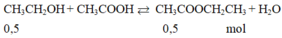
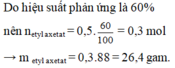
\(a) C_2H_5OH + CH_3COOH \buildrel{{H_2SO_4}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O\\ b) n_{CH_3COOH} = n_{C_2H_5OH} = \dfrac{9,2}{46} = 0,2(mol)\\ m_{CH_3COOH} = 0,2.60 = 12(gam)\\ c) n_{CH_3COOC_2H_5} = n_{C_2H_5OH} = 0,2(mol)\\ m_{CH_3COOC_2H_5} = 0,2.88 = 17,6(gam)\)