Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\\ KL:M\\ M+2HCl\rightarrow MCl_2+H_2\\ n_{MCl_2}=n_M=n_{H_2}=0,05\left(mol\right)\\ M_{MCl_2}=\dfrac{4,75}{0,05}=95\left(\dfrac{g}{mol}\right)\\ M\text{à}:M_{MCl_2}=M_M+71\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_M+71=95\\ \Leftrightarrow M_M=24\left(\dfrac{g}{mol}\right)\\ \Rightarrow M\left(II\right):Magie\left(Mg=24\right)\\ a=24.0,05=1,2\left(g\right)\)

\(n_{H_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(R+2HCl\rightarrow RCl_2+H_2\)
\(0.1........0.2................0.1\)
\(M_R=\dfrac{13.7}{0.1}=137\left(\dfrac{g}{mol}\right)\)
\(R:Ba\)
\(200\left(ml\right)=0.2\left(l\right)\)
\(C_{M_{HCl}}=\dfrac{0.2}{0.2}=1\left(M\right)\)

\(a.BTNT\left(H\right):n_{HCl}=2n_{H_2}=0,65\left(mol\right)\\ \Rightarrow CM_{HCl}=\dfrac{0,65}{0,5}=1,3M\\ b.2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ Đặt:\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\dfrac{3}{2}x+y=0,325\\27x+56y=9,65\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Al}=4,05\left(g\right)\\m_{Fe}=5,6\left(g\right)\end{matrix}\right.\)

Fe+2HCl->FeCl2+H2
x---2x-----------x
Mg+2HCl->MgCl2+H2
y------2y-----------y
Ta có :
\(\left\{{}\begin{matrix}56x+24y=24\\x+y=\dfrac{13,44}{22,4}\end{matrix}\right.\)
=>x=0,3 mol, y=0,3 mol
=>%m Fe=\(\dfrac{0,3.56}{24}.100\)=70%
=>%m Mg=100-70=30%
=>VHCl=\(\dfrac{0,3.2+0,3.2}{2}\)=0,6l=600ml
b)
XCl2+2AgNO3->2AgCl+X(NO3)2
0,6--------------------1,2mol
=>m AgCl=1,2.143,5=172,2g


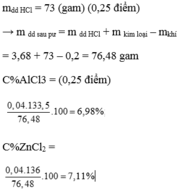
Ta có n(H2) = 6.72/22.4 = 0.3 (mol)
Ta có phương trình
2R + 6HCl --> 2RCl3 + 3H2
--> n(R) = 0.2 (mol)
---> M(R)= 5.4/0.2 =27 (g/mol)
--> R là Al
b) --> n (HCl) = 0.6 (mol)
--> m = 0.6*36.5*100/3.65=600(g)
m(AlCl3)= 0.2* 133.5=26.7(g)
XIN TRỢ GIÚP LÀM ƠN !!!!