Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al}=\dfrac{2,5}{27}=\dfrac{25}{270}=\dfrac{5}{54}\left(mol\right)\\ n_{H_2SO_4}=0,5\left(mol\right)\\ a,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ b,Vì:\dfrac{\dfrac{5}{54}}{2}< \dfrac{0,5}{4}\Rightarrow H_2SO_4dư\\ b,n_{H_2SO_4\left(dư\right)}=0,5-\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{13}{36}\left(mol\right)\\ \Rightarrow m_{H_2SO_4}=\dfrac{13}{36}.98=\dfrac{637}{18}\left(g\right)\\ c,n_{H_2}=\dfrac{3}{2}.n_{Al}=\dfrac{3}{2}.\dfrac{5}{54}=\dfrac{5}{36}\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=\dfrac{5}{36}.22,4=\dfrac{28}{9}\left(l\right)\)

PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
Làm gộp các phần còn lại
Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\) \(\Rightarrow\left\{{}\begin{matrix}n_{Al_2\left(SO_4\right)_3}=0,1mol\\n_{H_2SO_4}=n_{H_2}=0,3mol\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\m_{Al_2\left(SO_4\right)_3}=0,1\cdot342=34,2\left(g\right)\\m_{H_2SO_4}=0,3\cdot98=29,4\left(g\right)\end{matrix}\right.\)

a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Ta có: \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{2}>\dfrac{0,25}{6}\), ta được Al dư.
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{HCl}=0,125\left(mol\right)\Rightarrow V_{H_2}=0,125.22,4=2,8\left(l\right)\)
b, Theo PT: \(n_{Al\left(pư\right)}=\dfrac{1}{3}n_{HCl}=\dfrac{1}{12}\left(mol\right)\Rightarrow n_{Al\left(dư\right)}=0,1-\dfrac{1}{12}=\dfrac{1}{60}\left(mol\right)\)
\(\Rightarrow m_{Al}=\dfrac{1}{60}.27=0,45\left(g\right)\)

a.b.\(n_{Al}=\dfrac{5,4}{27}=0,2mol\)
\(n_{H_2SO_4}=\dfrac{39,2}{98}=0,4mol\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
Xét: \(\dfrac{0,2}{2}\) < \(\dfrac{0,4}{3}\) ( mol )
0,2 0,3 0,1 0,3 ( mol )
\(m_{H_2SO_4\left(dư\right)}=\left(0,4-0,3\right).98=9,8g\)
\(m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
c.\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
0,3 0,15 ( mol )
\(V_{kk}=V_{O_2}.5=\left(0,15.22,4\right).5=16,8l\)

PTHH: \(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
Ta có: \(\left\{{}\begin{matrix}n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\n_{HCl}=0,25\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,25}{2}\) \(\Rightarrow\) HCl còn dư, Kẽm p/ứ hết
\(\Rightarrow\left\{{}\begin{matrix}n_{H_2}=0,1\left(mol\right)\\n_{HCl\left(dư\right)}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=22,4\cdot0,1=2,24\left(l\right)\\m_{HCl\left(dư\right)}=0,05\cdot36,5=1,825\left(g\right)\end{matrix}\right.\)
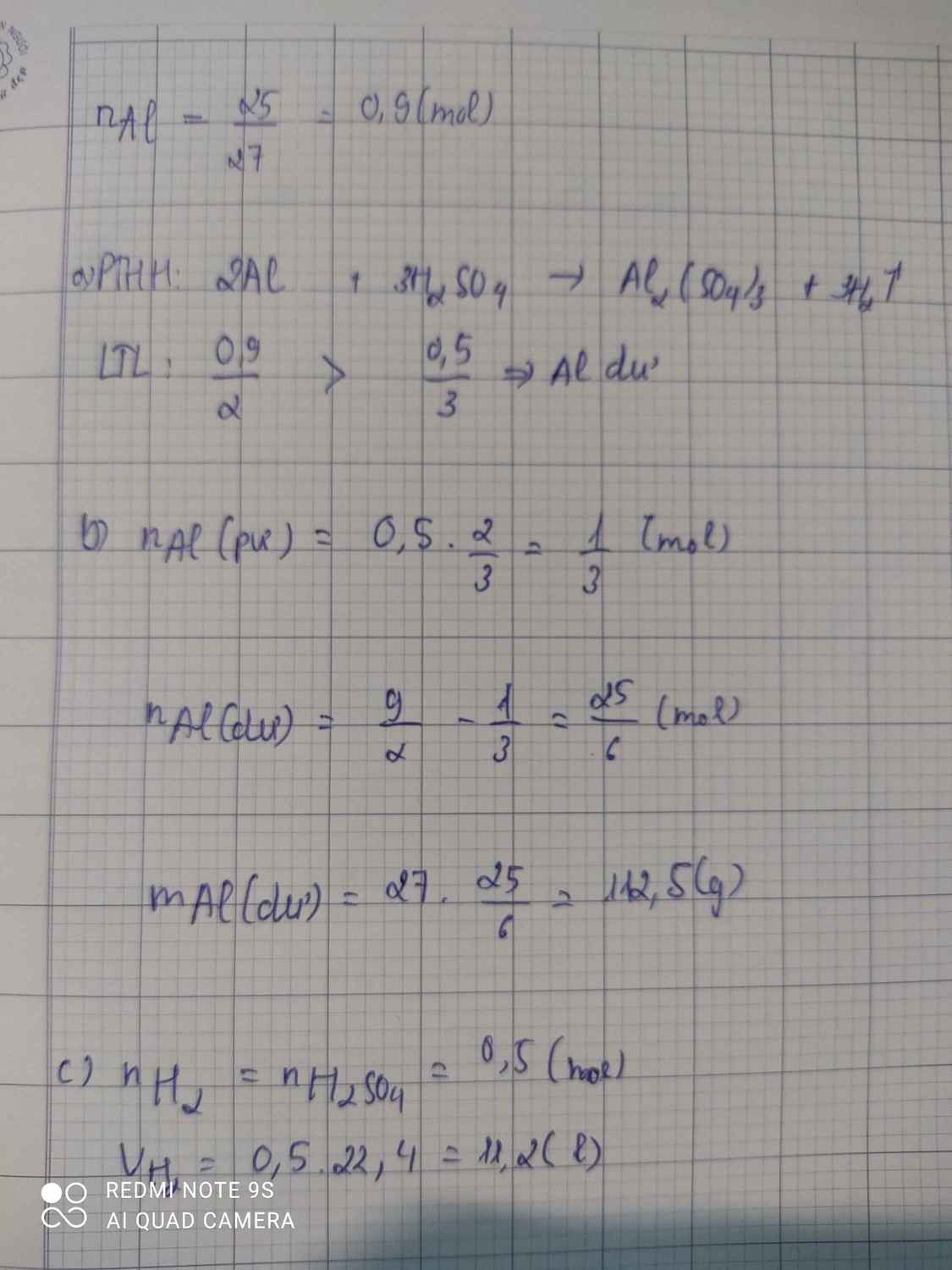

a: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
b: \(n_{Al}=\dfrac{2.5}{27}< \dfrac{1}{4}\)
=>H2SO4 dư, Al đủ
\(m_{H_2SO_4}=0.25\cdot98=24.5\left(g\right)\)
c: \(n_{Al_2\left(SO_4\right)_3}=\dfrac{2.5}{54}=\dfrac{5}{108}\left(mol\right)\)
\(\Leftrightarrow n_{H_2}=\dfrac{5}{36}\left(mol\right)\)
\(V_{H_2}=\dfrac{5}{36}\cdot22.4=\dfrac{28}{9}\left(lít\right)\)
Mình thấy bạn Thịnh tính lượng dư sai
Đây là bài mình từng làm, bạn tham khảo nhé!