Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Na_2SO_4}=\dfrac{71.20}{100.142}=0,1\left(mol\right)\)
\(n_{BaCl_2}=\dfrac{100.10,4}{100.208}=0,05\left(mol\right)\)
PTHH: \(Na_2SO_4+BaCl_2\rightarrow BaSO_4\downarrow+2NaCl\)
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,05}{1}\) => BaCl2 hết, Na2SO4 dư
PTHH: \(Na_2SO_4+BaCl_2\rightarrow BaSO_4\downarrow+2NaCl\)
0,05<--------0,05---->0,05------->0,1
=> \(\left\{{}\begin{matrix}m_{Na_2SO_4}=\left(0,1-0,05\right).142=7,1\left(g\right)\\m_{NaCl}=0,1.58,5=5,85\left(g\right)\end{matrix}\right.\)
mdd sau pư = 71 + 100 - 0,05.233 = 159,35(g)
=> \(\left\{{}\begin{matrix}C\%\left(Na_2SO_4\right)=\dfrac{7,1}{159,35}.100\%=4,456\%\\C\%\left(NaCl\right)=\dfrac{5,85}{159,35}.100\%=3,67\%\end{matrix}\right.\)

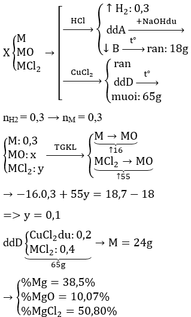
M + 2HCl → MCl2 + H2↑
MO + 2HCl → MCl2 + H2O
MCl2 + 2NaOH → M(OH)2↓ + 2NaCl
M(OH)2 → MO + H2O
M + CuCl2 → MCl2 + Cu↓

a) mNaOH= 200.20%= 40(g)
=>nNaOH=1(mol)
PTHH: 2 NaOH + CuCl2 -> 2 NaCl + Cu(OH)2
Dung dịch sau khi lọc kết tủa có NaCl.
nNaCl=nNaOH= 1(mol)
nCuCl2=nCu(OH)2=nNaOH/2=1/2=0,5(mol)
mNaCl=1.58,5=58,5(g)
mCuCl2=0,5.135=67,5(g)
=> mddCuCl2=(67,5.100)/10=675(g)
mCu(OH)2=0,5.98=49(g)
=>mddNaCl=mddNaOH+ mddCuCl2 - mCu(OH)2= 200+675 - 98=777(g)
=> \(C\%ddNaCl=\dfrac{58,5}{777}.100\approx7,529\%\)
b) PTHH: Cu(OH)2 -to-> CuO + H2O
0,5__________________0,5(mol)
m(rắn)=mCuO=0,5.80=4(g)

Bài 10:
PTHH: \(Na_2CO_3+BaCl_2\rightarrow2NaCl+BaCO_3\downarrow\)
a) Ta có: \(n_{Na_2CO_3}=\dfrac{200\cdot10,6\%}{106}=0,2\left(mol\right)=n_{BaCO_3}\)
\(\Rightarrow m_{BaCO_3}=0,2\cdot197=39,4\left(g\right)\)
b) Theo PTHH: \(n_{BaCl_2}=n_{BaCO_3}=0,2mol\)
\(\Rightarrow C\%_{BaCl_2}=\dfrac{0,2\cdot208}{120}\cdot100\%\approx34,67\%\)
c) Theo PTHH: \(n_{NaCl}=2n_{BaCl_2}=0,4mol\) \(\Rightarrow m_{NaCl}=0,4\cdot58,5=23,4\left(g\right)\)
Mặt khác: \(m_{dd}=m_{ddNa_2CO_3}+m_{ddBaCl_2}-m_{BaCO_3}=280,6\left(g\right)\)
\(\Rightarrow C\%_{NaCl}=\dfrac{23,4}{280,6}\cdot100\%\approx8,34\%\)
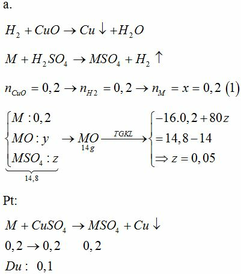
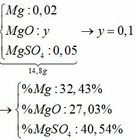

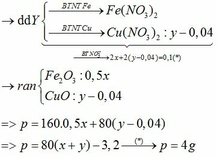
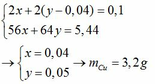
BaCl2 + Na2SO4 --> BaSO4 +2NaCl (1)
mBaCl2 =20,8(g) => nBaCl2=0,1(mol)
mNa2SO4=0,1m(g)
áp dụng định luật bảo toàn khối lượng ta có :
mBaSO4= 20,8 +0,1m - 13,12 =(7,68 + 0,1m) (g)
nBaSO4=\(\dfrac{7,68+0,1m}{233}\)(mol)
theo (1) : nBaSO4=nBaCl2=0,1(mol)
=>\(\dfrac{7,68+0,1m}{233}=0,1=>m=156,2\left(g\right)\)