Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{hhkhí}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\\ m_{tăng}=m_{C_2H_4}=4,2\left(g\right)\\ n_{C_2H_4}=\dfrac{4,2}{28}=0,15\left(mol\right)\\ n_{CH_4}=0,35-0,15=0,2\left(mol\right)\\ \left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,15}{0,35}=42,85\%\\\%V_{CH_4}=100\%-42,85\%=57,15\%\end{matrix}\right.\)
PTHH:
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,15 ------------------> 0,3
CH4 + O2 --to--> CO2 + 2H2O
0,2 -----------------> 0,2
Ca(OH)2 + CO2 ---> CaCO3 + H2O
0,5 -------> 0,5
\(m_{CaCO_3}=0,5.100=50\left(g\right)\)

\(a,n_{hhkhí\left(C_2H_4,C_2H_2\right)}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{Br_2}=\dfrac{80}{160}=0,5\left(mol\right)\\ Gọi\left\{{}\begin{matrix}n_{C_2H_4}=a\left(mol\right)\\n_{C_2H_2}=b\left(mol\right)\end{matrix}\right.\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Mol:a\rightarrow a\\ C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ Mol:b\rightarrow2b\\ Hệ.pt\left\{{}\begin{matrix}a+b=0,3\\a+2b=0,5\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,2\left(mol\right)\end{matrix}\right.\\ \%V_{C_2H_4}=\dfrac{0,1}{0,3}=33,33\%\\ \%V_{C_2H_2}=100\%-33,335=66,67\%\)
\(b,PTHH:\\ C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\\ Mol:0,1\rightarrow0,3\rightarrow0,2\\ 2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\\ Mol:0,2\rightarrow0,25\rightarrow0,4\\ n_{CO_2}=0,2+0,4=0,6\left(mol\right)\\ PTHH:Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\\ Mol:0,6\rightarrow0,6\rightarrow0,6\\ m_{CaCO_3}=0,6.100=60\left(g\right)\)

\(a,Gọi\left\{{}\begin{matrix}n_{CH_4}=a\left(mol\right)\\n_{C_2H_4}=b\left(mol\right)\\n_{C_2H_2}=c\left(mol\right)\end{matrix}\right.\\ n_{hhkhí}=0,4\left(mol\right)\\ n_{CO_2}=\dfrac{15,68}{22,4}=0,7\left(mol\right)\\ n_{Br_2}=\dfrac{64}{160}=0,4\left(mol\right)\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Mol:a\rightarrow a\\ C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ Mol:b\rightarrow2b\\ CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\\ Mol:a\rightarrow2a\rightarrow a\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\\ Mol:b\rightarrow3b\rightarrow2b\\ 2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\\ Mol:c\rightarrow2,5c\rightarrow2c\\ Hệ.pt\left\{{}\begin{matrix}a+b+c=0,4\\b+2c=0,4\\a+2b+2c=0,7\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,2\left(mol\right)\\c=0,1\left(mol\right)\end{matrix}\right.\)
\(\%V_{CH_4}=\%V_{C_2H_2}=\dfrac{0,1}{0,4}=25\%\\ \%V_{C_2H_4}=\dfrac{0,2}{0,4}=50\%\)
\(m_{CH_4}=0,1.16=1,6\left(g\right)\\ m_{C_2H_4}=28.0,2=5,6\left(g\right)\\ m_{C_2H_2}=0,1.26=2,6\left(g\right)\\ \%m_{CH_4}=\dfrac{1,6}{1,6+5,6+2,6}=16,32\%\\ \%m_{C_2H_4}=\dfrac{5,6}{1,6+5,6+2,6}=57,14\%\\ \%m_{C_2H_2}=100\%-16,32\%-57,14\%=26,54\%\)
\(b,PTHH:C_2H_5OH\rightarrow C_2H_4+H_2O\\ Mol:0,2\leftarrow0,2\\ m_{C_2H_5OH}=0,2.46=9,2\left(g\right)\)
Dài quá!!!

a) mtăng = mC2H4
=> \(n_{C_2H_4}=\dfrac{5,6}{28}=0,2\left(mol\right)\)
=> \(\%V_{C_2H_4}=\dfrac{0,2.22,4}{13,44}.100\%=33,33\%\)
\(\%V_{CH_4}=100\%-33,33\%=66,67\%\)
b) \(n_{CH_4}=\dfrac{13,44.66,67\%}{22,4}=0,4\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,4--------------->0,4
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,2----------------->0,4
Ca(OH)2 + CO2 --> CaCO3 + H2O
0,8----->0,8
=> mCaCO3 = 0,8.100 = 80 (g)
a.\(m_{tăng}=m_{C_2H_4}=5,6g\)
\(n_{hh}=\dfrac{13,44}{22,4}=0,6mol\)
\(n_{C_2H_4}=\dfrac{5,6}{28}=0,2mol\)
\(\%V_{C_2H_4}=\dfrac{0,2}{0,6}.100=33,33\%\)
\(\%V_{CH_4}=100\%-33,33\%=66,67\%\)
b.\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
0,2 0,4 ( mol )
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,4 0,4 ( mol )
\(n_{CO_2}=0,4+0,4=0,8mol\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
0,8 0,8 ( mol )
\(m_{CaCO_3}=0,8.100=80g\)

mtăng = mC2H4 = 2,8 (g)
=> \(n_{C_2H_4}=\dfrac{2,8}{28}=0,1\left(mol\right)\)
=> VC2H4 = 0,1.22,4 = 2,24 (l)
=> VCH4 = 4,48 - 2,24 = 2,24 (l)

Ta có: m dd Br2 tăng = mC2H4 = 2,8 (g)
\(\Rightarrow n_{C_2H_4}=\dfrac{2,8}{28}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{C_2H_4}=\dfrac{0,1.22,4}{3,36}.100\%\approx66,67\%\\\%V_{CH_4}\approx33,33\%\end{matrix}\right.\)
Có: \(n_{CH_4}=\dfrac{3,36}{22,4}-0,1=0,05\left(mol\right)\)
⇒ m hh = mCH4 + mC2H4 = 0,05.16 + 0,1.28 = 3,6 (g)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,05.16}{3,6}.100\%\approx22,22\%\\\%m_{C_2H_4}\approx77,78\%\end{matrix}\right.\)

a.\(m_{tăng}=m_{C_2H_4}=2,8g\)
\(V_{khí.thoát.ra}=V_{CH_4}+V_{CO_2}\)
\(n_{C_2H_4}=\dfrac{2,8}{28}=0,1mol\)
\(C_2H_4+3O_2\rightarrow\left(t^o\right)2CO_2+2H_2O\)
0,1 0,2 ( mol )
\(m_{H_2O\left(thu.được\right)}=0,2.18=3,6g\)
\(\Rightarrow m_{H_2O\left(pứCH_4\right)}=7,2-3,6=3,6g\)
\(n_{H_2O}=\dfrac{3,6}{18}=0,2mol\)
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
0,1 0,2 ( mol )
\(\Rightarrow V_{CH_4}=0,1.22,4=2,24l\)
\(\Rightarrow V_{CO_2}=3,36-2,24=1,12l\)
\(\Rightarrow V_{C_2H_4}=0,1.22,4=2,24l\)
\(\rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{2,24}{2,24+1,12+2,24}.100=40\%\\\%V_{CO_2}=\dfrac{1,12}{2,24+1,12+2,24}.100=20\%\\\%V_{C_2H_4}=100\%-40\%-20\%=40\%\end{matrix}\right.\)
b.\(\rightarrow\left\{{}\begin{matrix}m_{CH_4}=0,1.16=1,6g\\m_{CO_2}=0,05.44=2,2g\\m_{C_2H_4}=0,1.28=2,8g\end{matrix}\right.\)
\(\Rightarrow m_{hh}=1,6+2,2+2,8=6,6g\)
c.\(m_{PE}=28.n_{C_2H_4}.H\%=28.0,1.80\%=2,24g\)

a. Phương trình phản ứng :
C2H2 + 2Br2 → C2H2Br4 (1)
C2H4 + Br2 → C2H4Br2 (2)
b. Hỗn hợp khí B gồm có H2, C2H6. Gọi x, y ( mol ) lần lượt là số mol của H2 và C2H6 có trong 6,72 lít hỗn hợp B.
nB = x + y = 6,72 : 22,4 = 0,3 mol (I)
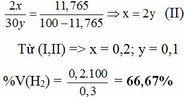
% V(C2H6) = 100% – 66,67% = 33,33%
c. nA = 11,2 : 22,4 = 0,5 mol , M A = 0,4 . 44 = 17,6 g/ mol
mA = 0,5 . 17,6 = 8,8 gam
mB = 0,2 . 2 + 0,1 . 30 = 3,4 gam
Vậy khối lượng bình Br2 tăng: m = mA – mB = 8,8 – 3,4 = 5,4 gam.

\(n_{\downarrow}=\dfrac{35}{100}=0,35mol\Rightarrow n_C=m_{CaCO_3}=0,35mol\)
\(\left\{{}\begin{matrix}CH_4:x\left(mol\right)\\C_2H_2:y\left(mol\right)\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x+y=\dfrac{4,48}{22,4}=0,2\\BTC:x+2y=0,35\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,05\\y=0,15\end{matrix}\right.\)
\(m_{tăng}=m_{Br_2}=2n_{C_2H_2}\cdot160=48g\)
\(\%V_{CH_4}=\dfrac{0,05}{0,05+0,15}\cdot100\%=25\%\)
\(\%V_{C_2H_2}=100\%-25\%=75\%\)
Gọi \(\left\{{}\begin{matrix}n_{CH_4}=x\\n_{C_2H_2}=y\end{matrix}\right.\)
\(n_{hh}=\dfrac{4,48}{22,4}=0,2mol\)
\(CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
x x ( mol )
\(2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\)
y 2y ( mol )
\(n_{CaCO_3}=\dfrac{35}{100}=0,35mol\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
0,35 0,35 ( mol )
Ta có:
\(\left\{{}\begin{matrix}x+y=0,2\\x+2y=0,35\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,05\\y=0,15\end{matrix}\right.\)
\(m_{tăng}=2m_{C_2H_2}=2.0,15.160=48g\)
\(V_{CH_4}=0,05.22,4=1,12l\)
\(V_{C_2H_2}=0,15.22,4=3,36l\)
\(a,n_{Br_2}=\dfrac{32}{160}=0,2\left(mol\right)\\ PTHH:C_2H_4+Br_2\rightarrow C_2H_4Br_2\\ Theo.pt:n_{C_2H_4}=n_{Br_2}=0,2\left(mol\right)\\ n_{hhkhi}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{CO_2}=0,3-0,2=0,1\left(mol\right)\\ m_{C_2H_4}=0,2.28=5,6\left(g\right)\\ m_{CO_2}=0,1.44=4,4\left(g\right)\\ b,C_{MddBr_2}=\dfrac{0,2}{0,5}=0,4M\)
ô