Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 6:
a) \(n_{O_2\left(tt\right)}=\dfrac{2,88}{24}=0,12\left(mol\right)\)
=> \(n_{O_2\left(PTHH\right)}=\dfrac{0,12.100}{80}=0,15\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,3<--------------------------------0,15
=> \(m_{KMnO_4}=0,3.158=47,4\left(g\right)\)
b) \(n_{KClO_3}=\dfrac{2,45}{122,5}=0,02\left(mol\right)\)
\(V_{O_2\left(tt\right)}=8.0,072=0,576\left(l\right)\)
=> \(n_{O_2\left(tt\right)}=\dfrac{0,576}{24}=0,024\left(mol\right)\)
PTHH: 2KClO3 --to--> 2KCl + 3O2
0,02---------------->0,03
=> nO2(hao hụt) = 0,03 - 0,024 = 0,006 (mol)
=> %O2 bị hao hụt = \(\dfrac{0,006}{0,03}.100\%=20\%\)

Câu 2:
\(4Al+3O2-->2Al2O3\)
a)\(n_{Al}=\frac{5,4}{27}=0,2\left(mol\right)\)
n\(_{O2}=\frac{6,72}{22,4}=0,3\left(mol\right)\)
\(n_{Al}\left(\frac{0,2}{4}\right)< nO2\left(\frac{0,3}{3}\right)\Rightarrow O2dư\)
n\(_{O2}=\frac{3}{4}n_{Al}=0,15\left(mol\right)\)
\(n_{O2}dư=0,3-0,15=0,15\left(mol\right)\)
m\(_{O2}dư=0,15.32=4,8\left(g\right)\)
b) \(n_{Al2O3}=\frac{1}{2}n_{Al}=0,15\left(mol\right)\)
\(m_{Al2O3}=0,15.102=15,3\left(g\right)\)

a, PT: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
Ta có: \(n_{Fe_3O_4}=\dfrac{4,64}{232}=0,02\left(mol\right)\)
Theo PT: \(\left\{{}\begin{matrix}n_{Fe}=3n_{Fe_3O_4}=0,06\left(mol\right)\\n_{O_2}=2n_{Fe_3O_4}=0,04\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,06.56=3,36\left(g\right)\)
\(m_{O_2}=0,04.32=1,28\left(g\right)\)
b, Phần này đề bài cho là KMnO4 hay KClO3 vậy bạn?

\(V_{O_2\left(thu.được\right)}=28=0,1=2,8\left(l\right)\)
=> \(V_{O_2\left(sinh.ra\right)}=\dfrac{2,8.100}{80}=3,5\left(l\right)\)
=> \(n_{O_2\left(sinh.ra\right)}=\dfrac{3,5}{22,4}=0,15625\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,3125<------------------------0,15625
=> mKMnO4 = 0,3125.158 = 49,375 (g)

a.\(n_{KClO_3}=\dfrac{m_{KClO_3}}{M_{KClO_3}}=\dfrac{12,25}{122,5}=0,1mol\)
\(2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\)
2 2 3 ( mol )
0,1 0,15
\(V_{O_2}=n_{O_2}.22,4=0,15.22,4=3,36l\)
b.\(V_{kk}=V_{O_2}.5=3,36.5=16,8l\)
c.\(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{28}{56}=0,5mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
3 2 1 ( mol )
0,5 > 0,15 ( mol )
0,225 0,15 ( mol )
\(m_{Fe\left(du\right)}=n_{Fe\left(du\right)}.M_{Fe}=\left(0,5-0,225\right).56=15,4g\)

\(n_{Al}=\dfrac{5.4}{27}=0,2mol\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
0,2 0,15 0,1
a)\(V_{O_2}=0,15\cdot22,4=3,36l\)
b)\(n_{O_2}=0,15\cdot10\%=0,015mol\)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,03 0,015
\(m_{KMnO_4}=0,03\cdot158=4,74g\)


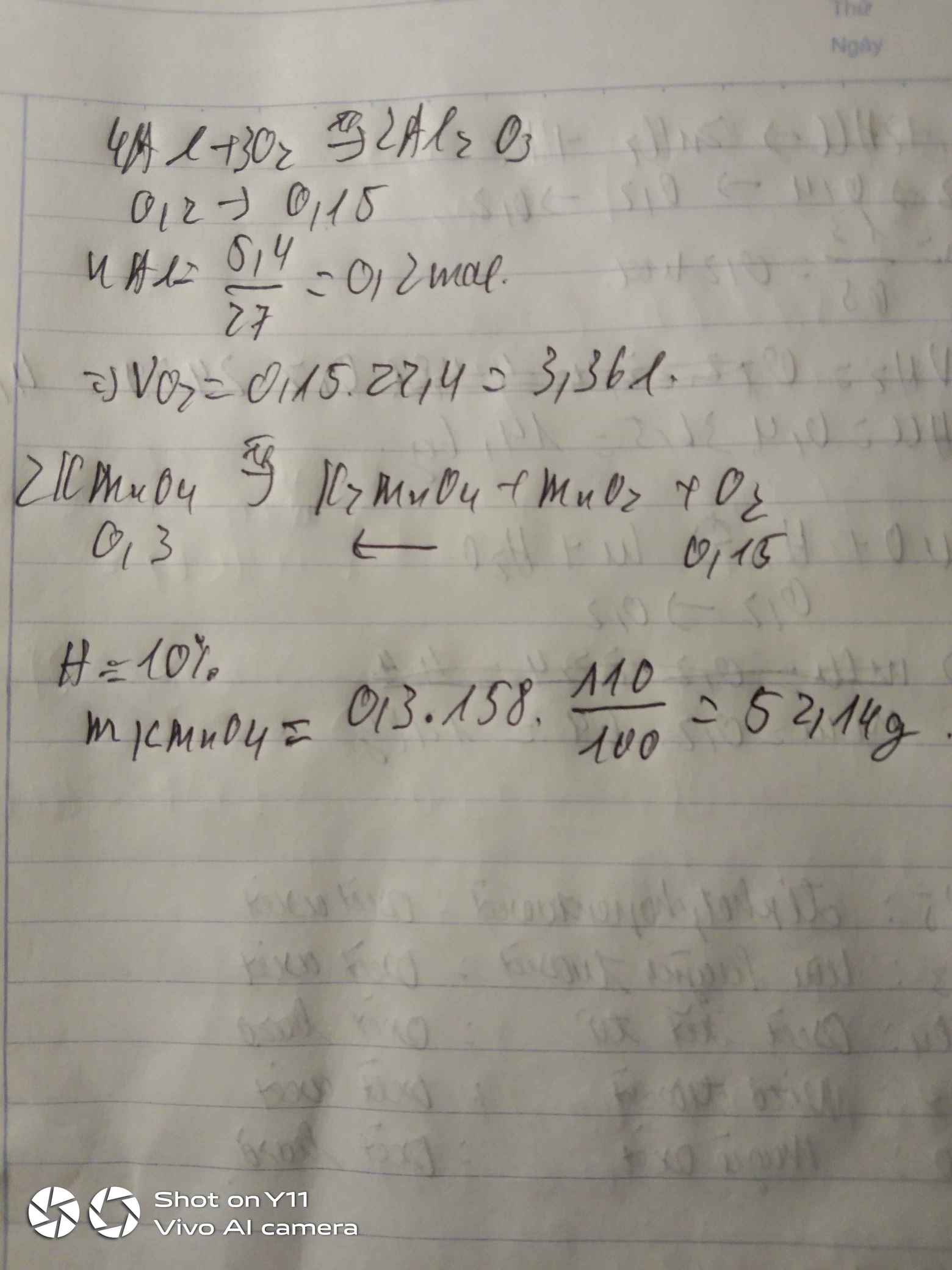
144cm^3 = 0,114 l
Số lượng oxi cần đun:
\(V_{O_2}=\frac{0,144}{40\%}=0,36\left(l\right)\)
\(n_{O_2}=\frac{V_{O_2}}{22,4}=\frac{0,36}{22,4}=0,016\left(mol\right)\)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
\(0,032\) \(0,016\) \(0,016\) \(0,016\) \(\left(mol\right)\)
\(m_{KMnO_4}=n_{KMnO_4}.M_{KMnO_4}=0,032.158=5,056\left(g\right)\)
nO2= \(\frac{0,144}{22,4}\)=\(\frac{9}{1400}\) mol
Vì O2 thu đc chỉ chiếm 60% lượng tạo thành nên thực tế đã tạo ra 3/280 mol O2
2KMnO4 \(\underrightarrow{^{to}}\) K2MnO4+ MnO2+ O2
\(\rightarrow\) nKMnO4=\(\frac{3}{140}\)mol
\(\rightarrow\)mKMnO4= 3,39g