Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) PTHH: \(MgO+2HCl\rightarrow MgCl_2+H_2O\)
b) Ta có: \(n_{MgO}=\dfrac{8}{40}=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{MgCl_2}=0,2\left(mol\right)\\n_{HCl}=0,4\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{MgCl_2}=0,2\cdot95=19\left(g\right)\\C\%_{HCl}=\dfrac{0,4\cdot36,5}{200}\cdot100\%=7,3\%\end{matrix}\right.\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{MgO}+m_{ddHCl}=208\left(g\right)\)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{19}{208}\cdot100\%\approx9,13\%\)
c) PTHH: \(MgCl_2+2NaOH\rightarrow2NaCl+Mg\left(OH\right)_2\downarrow\)
Ta có: \(\left\{{}\begin{matrix}n_{MgCl_2}=0,2\left(mol\right)\\n_{NaOH}=\dfrac{200\cdot4\%}{40}=0,2\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,2}{2}\) \(\Rightarrow\) NaOH p/ứ hết, MgCl2 còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{NaCl}=0,2\left(mol\right)\\n_{Mg\left(OH\right)_2}=0,1\left(mol\right)=n_{MgCl_2\left(dư\right)}\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{NaCl}=0,2\cdot58,5=11,7\left(g\right)\\m_{MgCl_2\left(dư\right)}=9,5\left(g\right)\\m_{Mg\left(OH\right)_2}=0,1\cdot58=5,8\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{ddA}+m_{ddNaOH}-m_{Mg\left(OH\right)_2}=402,2\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{NaCl}=\dfrac{11,7}{402,2}\cdot100\%\approx2,91\%\\C\%_{MgCl_2\left(dư\right)}=\dfrac{9,5}{402,2}\cdot100\%\approx2,36\%\end{matrix}\right.\)
Theo gt ta có: $n_{MgO}=0,2(mol)$
a, $MgO+2HCl\rightarrow MgCl_2+H_2O$
b, Ta có: $n_{HCl}=0,4(mol)\Rightarrow x=7,3$
Bảo toàn khối lượng ta có: $m_{ddA}=208(g)$
$\Rightarrow \%C_{MgCl_2}=9,13\%$
c, Ta có: $n_{NaOH}=0,2(mol)$
$\Rightarrow n_{Mg(OH)_2}=0,1(mol)$
Bảo toàn khối lượng ta có: $m_{ddB}=208+200-0,1.58=402,2(g)$
$\Rightarrow \%C_{MgCl_2}=2,36\%$

\(n_{Na}=0.02\left(mol\right)\)
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
\(0.02....................0.02........0.01\)
\(V_{H_2}=0.01\cdot22.4=0.224\left(l\right)\)
\(m_{NaOH}=0.02\cdot40=0.8\left(g\right)\)
\(C\%_{NaOH}=\dfrac{0.8}{0.46+200-0.01\cdot2}\cdot100\%=0.4\%\)

nHCl=0,6 mol
FeO+2HCl-->FeCl2+ H2O
x mol x mol
Fe2O3+6HCl-->2FeCl3+3H2O
x mol 2x mol
72x+160x=11,6 =>x=0,05 mol
A/ CFeCl2=0,05/0,3=1/6 M
CFeCl3=0,1/0,3=1/3 M
CHCl du=(0,6-0,4)/0,3=2/3 M
B/
NaOH+ HCl-->NaCl+H2O
0,2 0,2
2NaOH+FeCl2-->2NaCl+Fe(OH)2
0,1 0,05
3NaOH+FeCl3-->3NaCl+Fe(OH)3
0,3 0,1
nNaOH=0,6
CNaOH=0,6/1,5=0,4M

\(n_{MgCO_3}=\dfrac{16,8}{84}=0,2mol\\ a.MgCO_3+H_2SO_4->MgSO_4+H_2O+CO_2\\ 2NaOH+H_2SO_{\text{4 }}->Na_2SO_4+2H_2O\\ b.n_{H_2SO_4dư}=\dfrac{1}{2}n_{NaOH}=\dfrac{1}{2}.80.0,1:40=0,1mol\\ n_{H_2SO_4\left(MgCO_3\right)}=0,2mol\\ c.C\%=\dfrac{98.0,3}{200}.100\%=14,7\%\\ V=0,2.22,4=4,48L\\ d.m_{ddsau}=200+16,8-44.0,2+80=288g\\ C\%_{Na_2SO_4}=\dfrac{40.0,1}{288}.100\%=1,39\%\\ C\%_{MgSO_4}=\dfrac{120.0,2}{288}.100\%=8,33\%\)
\(n_{MgCO_3}=\dfrac{16,8}{84}=0,2\left(mol\right)\)
\(n_{NaOH}=\dfrac{80}{40}=2\left(mol\right)\)
PTHH :
\(MgCO_3+H_2SO_4\rightarrow MgSO_4+H_2O+CO_2\uparrow\)
0,2 0,2 0,2
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
2 1 1
Vậy có 0,2 mol H2SO4 phản ứng với MgCO3
có 1 mol H2SO4 phản ứng với NaOH
\(m_{H_2SO_4}=1,2.98=117,6\left(g\right)\)
\(c,C\%_{H_2SO_4}=\dfrac{117,6}{200}.100\%=58,8\%\)
\(V_{CO_2}=0,2.22,4=4,48\left(l\right)\)
\(d,m_{Na_2SO_4}=1.142=142\left(g\right)\)
\(m_{ddNaOH}=\dfrac{80.100}{10}=800\left(g\right)\)
\(m_{ddH_2SO_4dư}=1.98:58,8\%\approx166,67\left(g\right)\)
\(m_{ddNa_2SO_4}=800+166,67=966,67\left(g\right)\)
\(C\%_{Na_2SO_4}=\dfrac{142}{966,67}.100\%\approx14,69\%\)

Sửa đề 200ml dd HCl
\(a,n_{HCl}=0,2.0,1=0,02\left(mol\right)\)
PTHH: \(NaOH+HCl\rightarrow NaCl+H_2O\)
0,02<----0,02
\(C_{M\left(NaOH\right)}=\dfrac{0,02}{0,5}=0,04M\)
b) Muối thu được là NaCl phản ứng được với H2O, AgNO3
\(2NaCl+2H_2O\xrightarrow[\text{có màng ngăn}]{\text{điện phân}}2NaOH+Cl_2\uparrow+H_2\uparrow\\ NaCl+AgNO_3\rightarrow AgCl\downarrow+NaNO_3\)

\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,2 0,2 0,2 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{NaOH}=0,2\cdot40=8g\)
\(m_{ddNaOH}=4,6+0,2\cdot18-0,1\cdot2=8g\)
\(\Rightarrow C\%=\dfrac{m_{NaOH}}{m_{ddNaOH}}\cdot100\%=\dfrac{8}{8}\cdot100\%=100\%???\)
Sửa đề: Tính nồng độ mol của dung dịch NaOH???
\(C_{M_{NaOH}}=\dfrac{0,2}{0,3}=\dfrac{2}{3}M\)
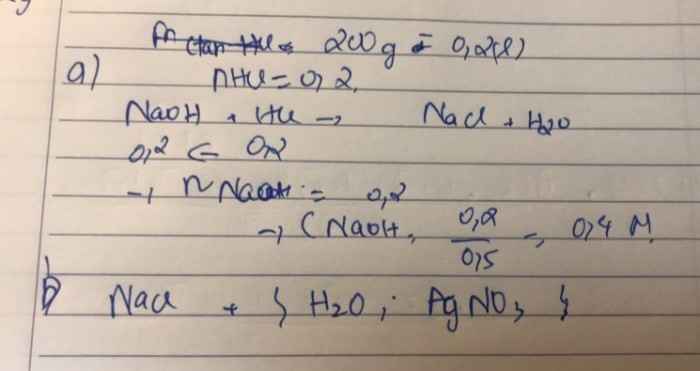
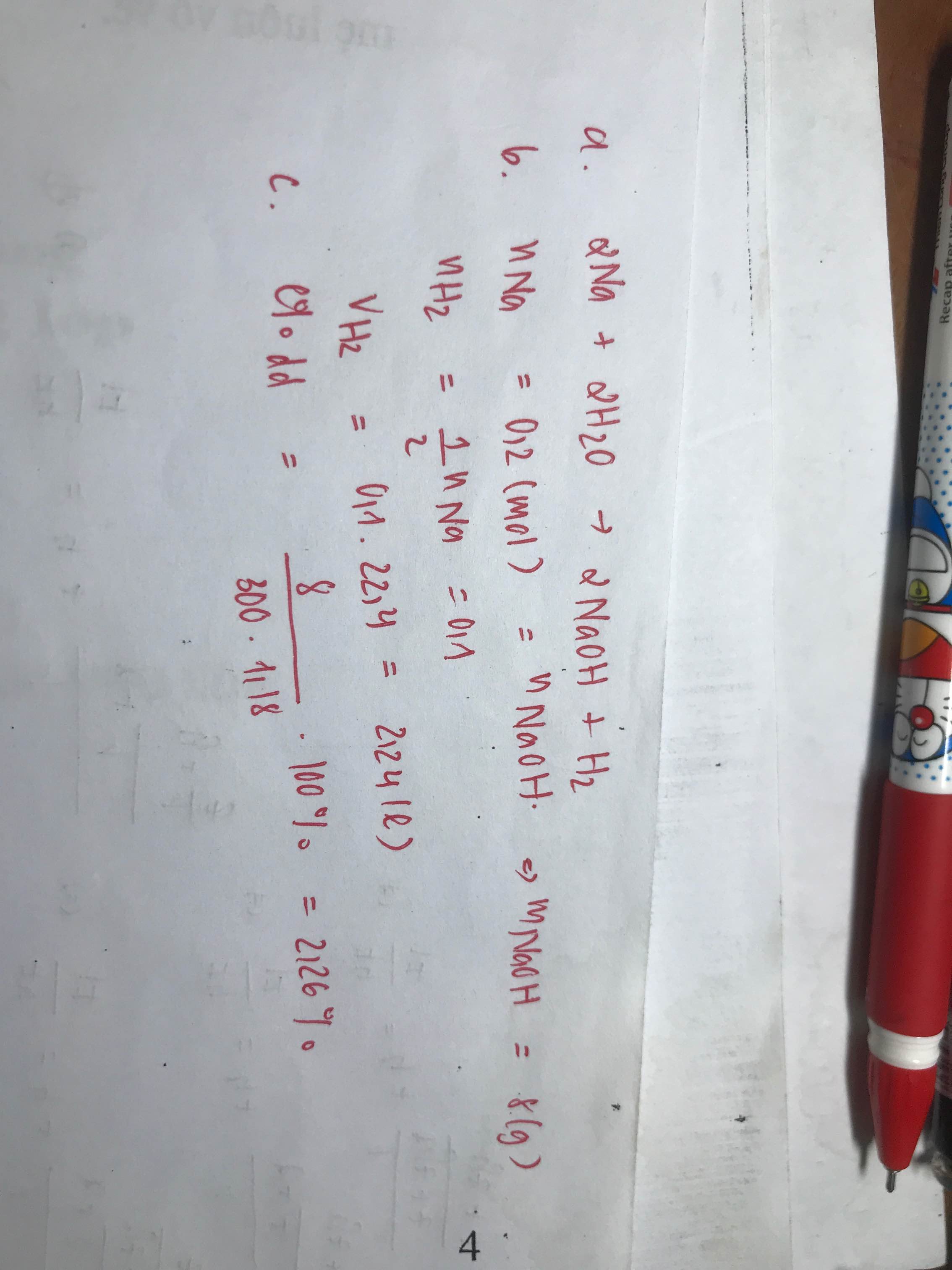
1/a/ Xem lại đề. Sao có chuyện hòa tan 20g NaOH vào 200g nước lại thu được 300g dung dịch.
b/ Câu b cũng xem lại đề luôn.
2/ \(m_{HCl}=200.5\%=10\left(g\right)\)
\(\Rightarrow n_{HCl}=\dfrac{10}{36,5}=\dfrac{20}{73}\left(mol\right)\)
\(2HCl\left(\dfrac{20}{73}\right)+Zn\left(\dfrac{10}{73}\right)\rightarrow ZnCl_2+H_2\left(\dfrac{10}{73}\right)\)
\(\Rightarrow m_{Zn}=65.\dfrac{10}{73}=\dfrac{650}{73}\left(g\right)\)
\(\Rightarrow V_{H_2}=\dfrac{10}{73}.22,4=\dfrac{224}{73}\left(l\right)\)
Bắt quả Kiên