Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(1)PTHH:CaCO_3\xrightarrow{t^o}CaO+CO_2\uparrow\\ n_{CaCO_3}=\dfrac{500.95\%}{100}=4,75(mol)\\ \Rightarrow n_{CaO}=4,75(mol)\\ \Rightarrow m_{CaO}=4,75.56=266(g)\\ \Rightarrow m_{CaO(tt)}=266.80\%=212,8(g)\\ m_{CaCO_3(k p/ứ)}=500.95\%.20\%=95(g)\\ \Rightarrow m_A=95+212,8=307,8(g)\\ 2)\%m_{CaO}=\dfrac{212,8}{307,8}.100\%=69,136\%\\ n_{CO_2}=n_{CaO}=4,75(mol)\\ \Rightarrow V_{CO_2}=4,75.22,4=106,4(l)\)

\(m_{CaCO_3}=500\cdot95\%=475\left(g\right)\)
\(BTKL:\)
\(m_{CO_2}=m_{CaCO_3}-m_B=475-332.8=142.2\left(g\right)\)
\(n_{CO_2}=\dfrac{142.2}{44}=\dfrac{711}{220}\left(mol\right)\)
\(CaCO_3\underrightarrow{^{t^0}}CaO+CO_2\)
\(n_{CaCO_3\left(pư\right)}=n_{CO_2}=\dfrac{711}{220}\left(mol\right)\)
\(H\%=\dfrac{\dfrac{711}{220}\cdot100}{475}\cdot100\%=68.04\%\)
Cho mình hỏi tính hiệu suất của phản ứng là tính hiệu suất của cái naof cũng được ạ

\(m_{CaCO_3}=90\%.400=360\left(g\right)\\ \rightarrow n_{CaCO_3}=\dfrac{360}{100}=3,6\left(mol\right)\)
PTHH: CaCO3 --to--> CaO + CO2
3,6 ----------> 3,6 -----> 3,6
\(\rightarrow n_{CaO}=3,6.75\%=2,7\left(mol\right)\\ \rightarrow n_{CaCO_3\left(chưa.pư\right)}=3,6-2,7=0,9\left(mol\right)\)
\(\rightarrow m_X=0,9.100+2,7.56=241,2\left(g\right)\\ \%m_{CaO}=\dfrac{0,9.100}{241,2}=37,31\%\)
\(V_Y=V_{CO_2}=3,6.75\%.22,4=60,48\left(l\right)\)
\(m_{CaCO_3}=\dfrac{400\cdot90\%}{100\%}=360g\Rightarrow n_{CaCO_3}=\dfrac{360}{100}=3,6mol\)
\(CaCO_3\underrightarrow{t^o}CaO+CO_2\)
3,6 3,6 3,6
Thực tế: \(n_{CaO}=3,6\cdot75\%=2,7mol\)
\(\Rightarrow m_{CaO}=2,7\cdot56=151,2g\)

a)mCaCO3=500.80%=400(g) -> nCaCO3=400/100=4(mol)
PTHH: CaCO3 -to-> CaO + H2O
nCaO(LT)=nCaCO3=4(mol)
=> nCaO(TT)=4. 70%=2,8(mol)
=>mX=mCaO+ m(trơ)+ mCaCO3(chưa p.ứ)=2,8.56+100+ 1,2.100=376,8(g)
b) %mCaO= (156,8/376,8).100=41,614%

Câu 7:
\(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\\ Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ Vì:\dfrac{0,4}{1}>\dfrac{0,3}{1}\Rightarrow H_2SO_4dư,Znhết\\ a,n_{H_2}=n_{Zn}=0,3\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\\ b,n_{O\left(mất\right)}=n_{H_2O}=n_{H_2}=0,3\left(mol\right)\\ \Rightarrow m_{giảm}=m_{O\left(mất\right)}=0,3.16=4,8\left(g\right)\\ \Rightarrow m=4,8\left(g\right)\)
Câu 6:
- Giả sử có 1 mol hỗn hợp khí A.
\(\Rightarrow\left\{{}\begin{matrix}n_{N_xO}=30\%.1=0,3\left(mol\right)\\n_{SO_2}=30\%.1=0,3\left(mol\right)\\n_{CO_2}=1-\left(0,3+0,3\right)=0,4\left(mol\right)\end{matrix}\right.\\ \Rightarrow m_A=0,3.\left(14x+16\right)+0,3.64+0,4.44=41,6+4,2x\left(g\right)\\ \%m_{N_xO}=19,651\%\\ \Leftrightarrow\dfrac{4,2x+4,8}{41,6+4,2x}.100\%=19,651\%\\ \Leftrightarrow x=1\\ \Rightarrow N_xO.là:NO\\ M_{hhA}=\dfrac{0,3.30+0,3.64+0,4.44}{1}=45,8\left(\dfrac{g}{mol}\right)\\ \Rightarrow d_{\dfrac{hhA}{H_2}}=\dfrac{45,8}{2}=22,9\)

mrắn (sau khi nung) = \(\dfrac{300.78}{100}=234\left(g\right)\)
=> mCO2 = 300 - 234 = 66 (g)
=> \(n_{CO_2}=\dfrac{66}{44}=1,5\left(mol\right)\)
mCaCO3(bđ) = 300.80% = 240 (g)
PTHH: CaCO3 --to--> CaO + CO2
1,5<-----------------1,5
=> \(H\%=\dfrac{1,5.100}{240}.100\%=62,5\%\)

\(m_{CaCO_3}=400\cdot90\%=360\left(g\right)\)
\(m_{trơ}=400-360=40\left(g\right)\)
\(n_{CaCO_3}=\dfrac{360}{100}=3.6\left(mol\right)\)
\(a.\)
\(n_{CaCO_3\left(pư\right)}=3.6\cdot75\%=2.7\left(mol\right)\)
\(CaCO_3\underrightarrow{^{^{t^0}}}CaO+CO_2\)
\(2.7........2.7...........2.7\)
\(m_X=m_{CaO}+m_{CaCO_3\left(dư\right)}+m_{trơ}=2.7\cdot56+\left(3.6-2.7\right)\cdot100+40=281.2\left(g\right)\)
\(b.\)
\(\%CaO=\dfrac{2.7\cdot56}{281.2}\cdot100\%=53.77\%\)
\(V_{CO_2}=2.7\cdot22.4=60.48\left(l\right)\)
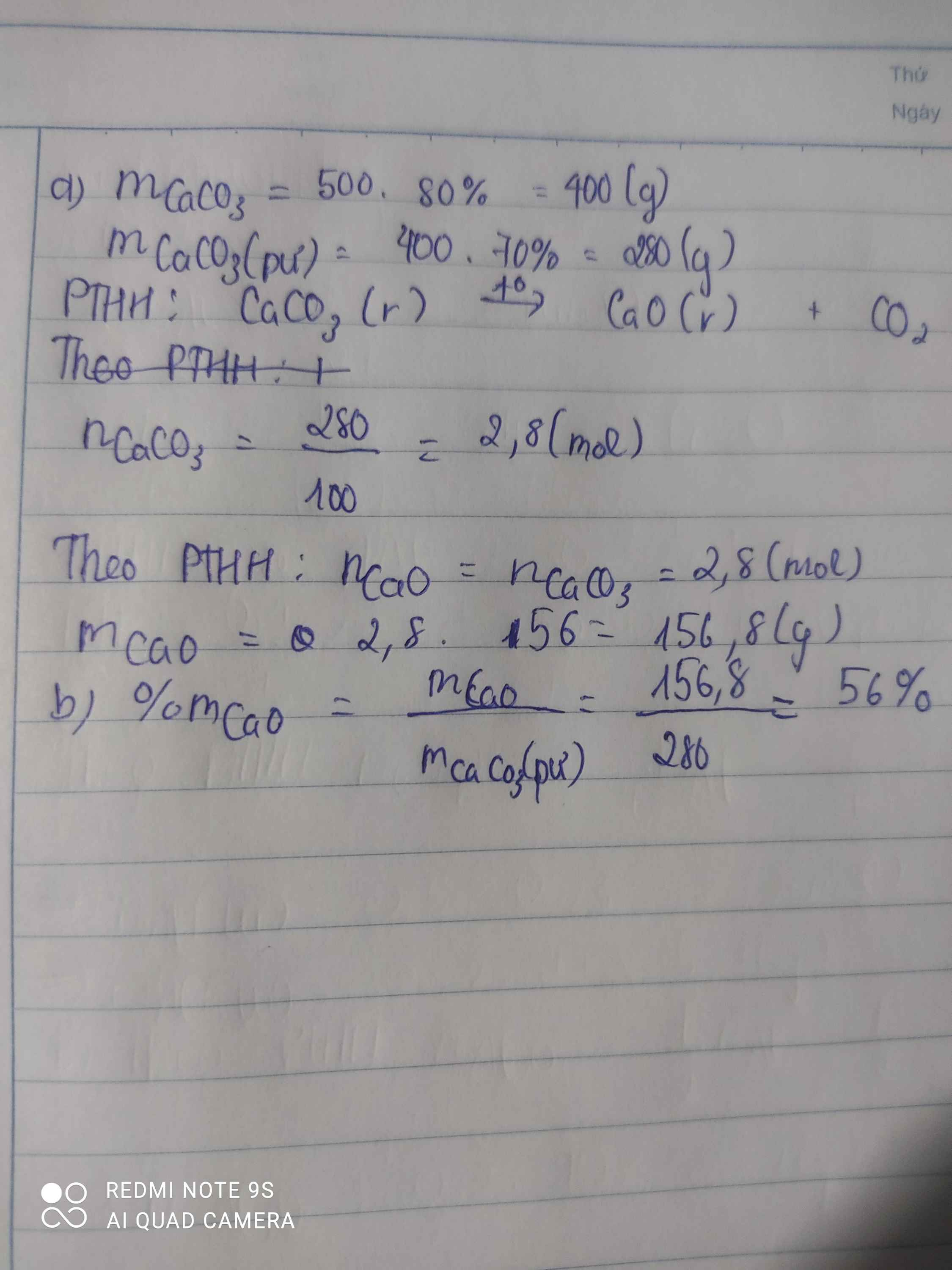
1)
1,2 tấn = 1200(kg)
5 tạ = 500(kg)
\(m_{CaCO_3} = 1200.80\% = 960(kg)\)
\(CaCO_3 \xrightarrow{t^o} CaO + CO_2\\ n_{CaCO_3\ pư} = n_{CaO} = \dfrac{500}{56}(mol)\\ \Rightarrow H = \dfrac{\dfrac{500}{56}.100}{960}.100\% = 93\%\)
1 (H)= 93,11%
2 (H)=88.08%
m cao=1.064(tấn)
==> m cr = 1.065(tấn)
%m cao = 56%