
 Giúp mình câu 9 và 31
Giúp mình câu 9 và 31
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



Câu 1: \(x\sqrt{\dfrac{y^2}{x}}=\sqrt{\dfrac{y^2}{x^3}}\)

31)
BTNT C: \(n_C=n_{CO_2}=n_{BaCO_3}=\dfrac{59,1}{197}=0,3\left(mol\right)\)
Ta có: \(m_{gi\text{ảm}}=m_{BaCO_3}-m_{CO_2}-m_{H_2O}\)
\(\Rightarrow59,1-0,3.44-m_{H_2O}=38,7\Leftrightarrow m_{H_2O}=7,2\left(g\right)\\ \Rightarrow n_{H_2O}=\dfrac{7,2}{18}=0,4\left(mol\right)\Rightarrow n_H=2n_{H_2O}=0,8\left(mol\right)\)
Ta có: \(n_{H_2O}>n_{CO_2}\left(0,4>0,3\right)\Rightarrow X\) thuộc dãy đồng đẳng ankan
Đặt CTPT của X là \(C_nH_{2n+2}\left(n\in N;n\ge1\right)\)
Ta có: \(n_X=n_{H_2O}-n_{CO_2}=0,1\left(mol\right)\)
Mà \(m_X=0,3.12+0,8=4,4\left(g\right)\)
\(\Rightarrow M_X=\dfrac{4,4}{0,1}=44\left(g/mol\right)\\ \Rightarrow14n+2=44\Leftrightarrow n=3\left(t/m\right)\)
Vậy X là C3H8 => Chọn C

\(\left\{{}\begin{matrix}\overrightarrow{GA}+\overrightarrow{GB}+\overrightarrow{GC}+\overrightarrow{GD}=0\\\overrightarrow{G_0B}+\overrightarrow{G_0C}+\overrightarrow{G_0D}=0\end{matrix}\right.\)
\(\overrightarrow{GA}+\overrightarrow{GB}+\overrightarrow{GC}+\overrightarrow{GD}=\overrightarrow{0}\)
\(\Leftrightarrow\left(\overrightarrow{GG_0}+\overrightarrow{G_0A}\right)+\left(\overrightarrow{GG_0}+\overrightarrow{G_0B}\right)+\left(\overrightarrow{GG_0}+\overrightarrow{G_0C}\right)+\left(\overrightarrow{GG_0}+\overrightarrow{G_0D}\right)=\overrightarrow{0}\)
\(\Leftrightarrow4\overrightarrow{GG_0}+\overrightarrow{G_0A}+\left(\overrightarrow{G_0B}+\overrightarrow{G_0C}+\overrightarrow{G_0D}\right)=\overrightarrow{0}\)
\(\Rightarrow\overrightarrow{AG_0}=4\overrightarrow{GG_0}\)
\(\Rightarrow\overrightarrow{AG}+\overrightarrow{GG_0}=4\overrightarrow{GG_0}\)
\(\Rightarrow\overrightarrow{AG}=3\overrightarrow{GG_0}\)
\(\Rightarrow\overrightarrow{GA}=-3\overrightarrow{GG_0}\)

Câu 31:
PT: \(CH_2=CH_2+H_2O\underrightarrow{t^o,xt}CH_3CH_2OH\)
→ Đáp án: A
Câu 32:
Ta có: \(n_{CO_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
\(n_X=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
Gọi CTPT của X là CnH2n.
\(\Rightarrow n=\dfrac{n_{CO_2}}{n_X}=5\)
Vậy: X là C5H10.
→ Đáp án: D

30.
\(V_{\left(O;\dfrac{3}{4}\right)}\left(M\right)=M_1\Rightarrow\left\{{}\begin{matrix}x_1=\dfrac{3}{4}.4=3\\y_1=\dfrac{3}{4}.1=\dfrac{3}{4}\end{matrix}\right.\)
\(Q_{\left(O;-90^0\right)}\left(M_1\right)=M'\Rightarrow\left\{{}\begin{matrix}x'=y_1=\dfrac{3}{4}\\y'=-x_1=-3\end{matrix}\right.\)
\(\Rightarrow M'\left(\dfrac{3}{4};-3\right)\)
31.
\(cos\dfrac{x}{2}=-\dfrac{\sqrt{3}}{2}\Rightarrow\dfrac{x}{2}=\pm\dfrac{5\pi}{6}+k2\pi\)
\(\Rightarrow x=\pm\dfrac{5\pi}{3}+k4\pi\)
Nghiệm dương nhỏ nhất \(x=\dfrac{5\pi}{3}\Rightarrow a+b=5+3=8\)






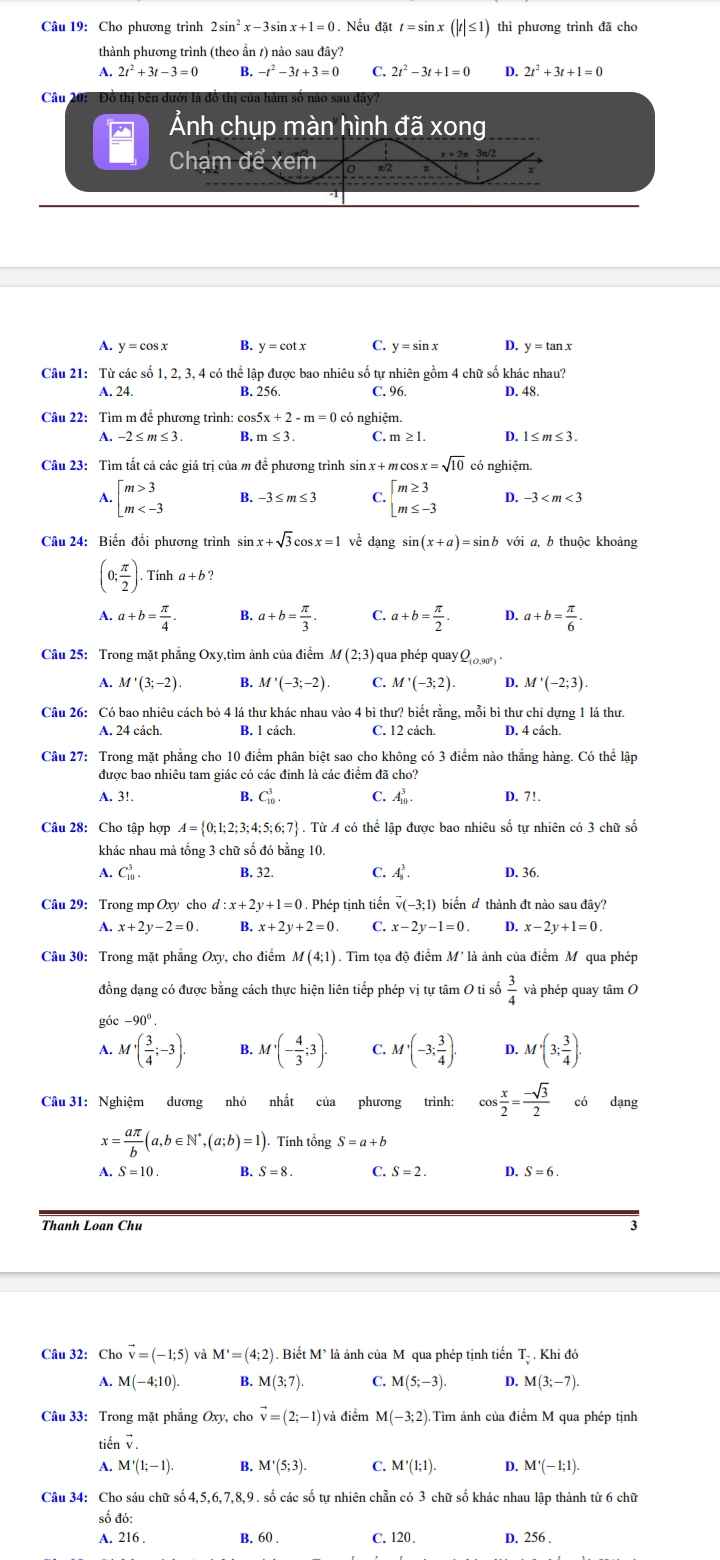


Câu 9:
\(94n_{C_6H_5OH}+46n_{C_2H_5OH}=13,04\left(g\right)\left(1\right)\)
PT: \(C_6H_5OH+Na\rightarrow C_6H_5ONa+\dfrac{1}{2}H_2\)
\(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{C_6H_5OH}+\dfrac{1}{2}n_{C_2H_5OH}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{C_6H_5OH}=0,08\left(mol\right)\\n_{C_2H_5OH}=0,12\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\%n_{C_2H_5OH}=\dfrac{0,12}{0,12+0,08}.100\%=60\%\)
Đáp án: B
Câu 31:
Ta có: m tinh bột = 1000.80% = 800 (kg) \(\Rightarrow n_{\left(C_6H_{10}O_5\right)_n}=\dfrac{800}{162n}=\dfrac{400}{81n}\left(kmol\right)\)
PT: \(\left(C_6H_{10}O_5\right)_n+nH_2O\underrightarrow{H^+,t^o}nC_6H_{12}O_6\)
\(C_6H_{12}O_6\underrightarrow{t^o,xt}2C_2H_5OH+2CO_2\)
\(\Rightarrow n_{C_2H_5OH\left(LT\right)}=2n_{C_6H_{12}O_6}=2n.n_{\left(C_6H_{10}O_5\right)_n}=\dfrac{800}{81}\left(kmol\right)\)
Mà: H = 70% \(\Rightarrow n_{C_2H_5OH\left(TT\right)}=\dfrac{800}{81}.70\%=\dfrac{560}{81}\left(kmol\right)\)
\(\Rightarrow m_{C_2H_5OH\left(TT\right)}=\dfrac{560}{81}.46=\dfrac{25760}{81}\left(kg\right)\)
\(\Rightarrow V_{C_2H_5OH}=\dfrac{\dfrac{25760}{81}}{0,8}=\dfrac{32200}{81}\left(l\right)\)
\(\Rightarrow V_{C_2H_5OH\left(60^o\right)}=\dfrac{\dfrac{32200}{81}}{0,6}\approx662,55\left(l\right)\)
Đáp án: A