Câu 14. Từ 36 g glucozơ lên men rượu thì thu được bao nhiêu lit dd rượu 5,750. Biết H% pư = 80%, Drượu = 0,8 (g/ml).
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(m_{C_2H_5OH\left(nguyên.chất\right)}=\dfrac{0,5.30}{100}=0,15l=150ml\)
\(\rightarrow m_{H_2O}=500-150=350ml\)
\(m_{C_2H_5OH}=150.0,8=120g\)
\(m_{H_2O}=350.1=350g\)
\(\left\{{}\begin{matrix}n_{C_2H_5OH}=\dfrac{120}{46}=2,6mol\\n_{H_2O}=\dfrac{350}{18}=19,44mol\end{matrix}\right.\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
2,6 1,3 ( mol )
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
19,44 9,72 ( mol )
\(V_{H_2}=\left(1,3+9,72\right).22,4=246,848l\)
\(0,5lít=500ml\)
\(m_{C_2H_5OH}=500.0,8=400g\)
\(n_{C_2H_5OH}=\dfrac{400}{46}=8,69mol\)
\(n_{Na}=\dfrac{300}{23}=12,04mol\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
8,69 < 12,04 ( mol )
8,69 8,69 ( mol )
\(V_{H_2}=8,96.22,4=200,704l\)

\(a,n_{C_6H_{12}O_6}=\dfrac{36}{180}=0,2\left(mol\right)\)
PTHH: \(C_6H_{12}O_6\underrightarrow{\text{men rượu}}2C_2H_5OH+2CO_2\uparrow\)
0,2----------------->0,4----------->0,4
=> VCO2 = 0,4.22,4 = 8,96 (l)
b, mC2H5OH = 0,4.46.50% = 9,2 (g)
\(c,V_{C_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\\ \rightarrow V_{ddC_2H_5OH}=\dfrac{11,5.100}{60}=\dfrac{115}{6}\left(ml\right)\)

mC2H5OH(bd) = 0,8.23 = 18,4 (g)
=> \(n_{C_2H_5OH\left(bd\right)}=\dfrac{18,4}{46}=0,4\left(mol\right)\)
=> \(n_{C_2H_5OH\left(pư\right)}=\dfrac{0,4.75}{100}=0,3\left(mol\right)\)
PTHH: C2H5OH --H2SO4,170oC--> C2H4 + H2O
0,3------------------------->0,3
=> VC2H4 = 0,3.22,4 = 6,72 (l)

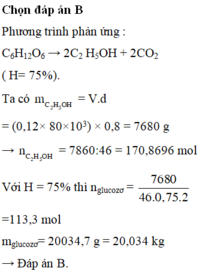

\(n_{C_6H_{12}O_6}=\dfrac{36}{180}=0,2\left(mol\right)\)
PTHH: C6H12O6 \(\xrightarrow{\text{men rượu}}\) 2CO2 + 2C2H5OH
0,2 ------------------------------> 0,4
\(\rightarrow m_{C_2H_5OH}=0,4.80\%.46=14,72\left(g\right)\\ \rightarrow V_{C_2H_5OH}=\dfrac{14,72}{0,8}=18,4\left(g\right)\\ \rightarrow V_{ddC_2H_5OH}=\dfrac{18,4.100}{5,75}=320\left(ml\right)\)