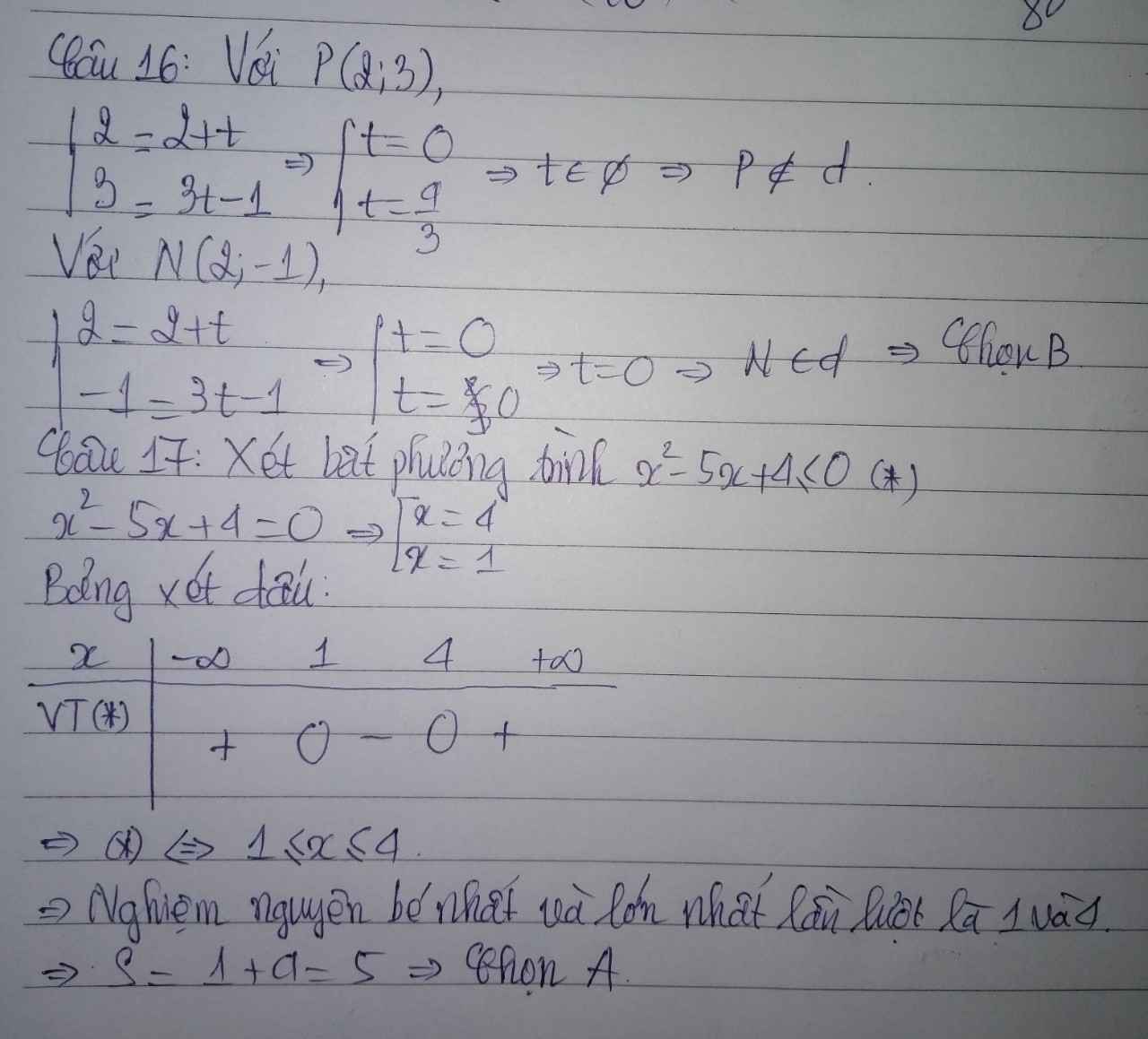Cho mình hỏi bài 16,17 với ạ .mình cảm ơn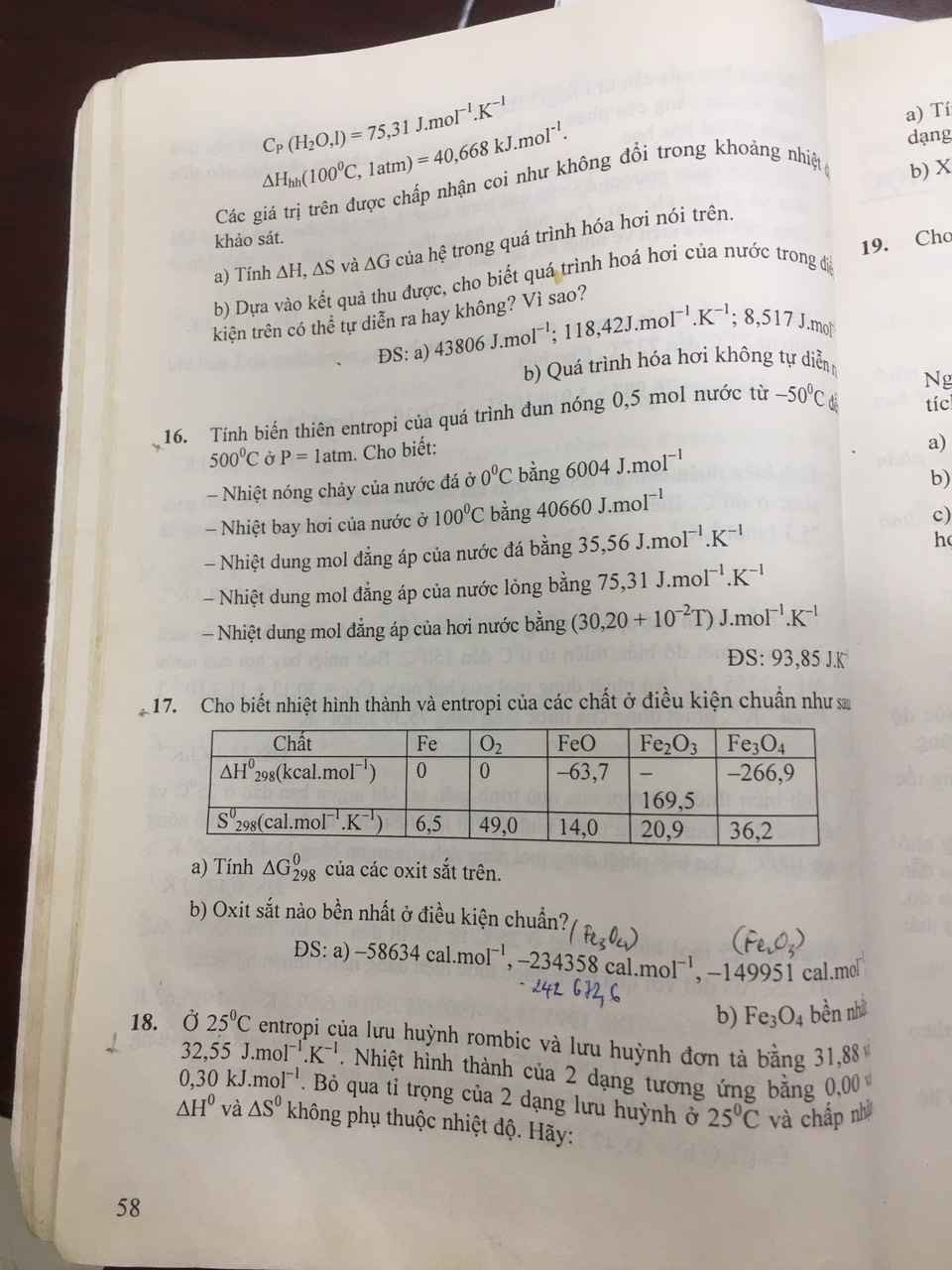
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.





3.
Do M là trung điểm BC \(\Rightarrow\overrightarrow{CM}=\dfrac{1}{2}\overrightarrow{CB}\)
N là trung điểm AC \(\Rightarrow\overrightarrow{AN}=\dfrac{1}{2}\overrightarrow{AC}\)
K là trung điểm AB \(\Rightarrow\overrightarrow{BK}=\dfrac{1}{2}\overrightarrow{BA}\)
Do đó:
\(\overrightarrow{AN}+\overrightarrow{CM}-\overrightarrow{KB}=\overrightarrow{AN}+\overrightarrow{CM}+\overrightarrow{BK}=\dfrac{1}{2}\overrightarrow{AC}+\dfrac{1}{2}\overrightarrow{CB}+\dfrac{1}{2}\overrightarrow{BA}\)
\(=\dfrac{1}{2}\overrightarrow{AB}+\dfrac{1}{2}\overrightarrow{BA}=\overrightarrow{0}\)
4.
\(\overrightarrow{BC}=\left(6;-2\right)\)
Gọi \(A'\left(x;y\right)\Rightarrow\overrightarrow{BA'}=\left(x+3;y-1\right)\)
Do A' thuộc BC \(\Rightarrow\overrightarrow{BA'}\) và \(\overrightarrow{BC}\) cùng phương
\(\Rightarrow\dfrac{x+3}{6}=\dfrac{y-1}{-2}\Rightarrow x=-3y\)
\(\Rightarrow A'\left(-3y;y\right)\Rightarrow\overrightarrow{AA'}=\left(-3y-2;y-4\right)\)
Mà AA' vuông góc BC \(\Rightarrow\overrightarrow{AA'}.\overrightarrow{BC}=0\)
\(\Rightarrow6\left(-3y-2\right)-2\left(y-4\right)=0\Rightarrow y=-\dfrac{1}{5}\)
\(\Rightarrow A'\left(\dfrac{3}{5};-\dfrac{1}{5}\right)\)

\(B=2+2^2+2^3+2^4+...+2^{99}+2^{100}=2\left(1+2^2+2^3+2^4\right)+...+2^{96}\left(1+2^2+2^3+2^4\right)=2.31+2^6.31+...+2^{96}.31=31\left(2+2^6+...+2^{96}\right)⋮31\)


Bài 1:
(1) \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
(2) \(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
(3) \(AlCl_3+3KOH\rightarrow3KCl+Al\left(OH\right)_3\downarrow\)
(4) \(Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2O\)
(5) \(2Al\left(OH\right)_3\xrightarrow[]{t^o}Al_2O_3+3H_2O\)
(6) \(Al\left(OH\right)_3+NaOH\rightarrow NaAlO_2+2H_2O\)
(7) \(Al_2O_3+2NaOH\rightarrow2NaAlO_2+H_2O\)
(8) \(Al+NaOH+H_2O\rightarrow NaAlO_2+\dfrac{3}{2}H_2\uparrow\)
(9) \(2Al_2O_3\xrightarrow[criolit]{đpnc}4Al+3O_2\)
Bài 2:
PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
a_______a_______a_____a (mol)
\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\)
b_______b________b____b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}56a+24b=21,6\\a+b=\dfrac{11,2}{22,4}=0,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,3\\b=0,2\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,3\cdot56}{21,6}\cdot100\%\approx77,78\%\\\%m_{Mg}=22,22\%\end{matrix}\right.\)
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{Mg\left(OH\right)_2}=n_{Mg}=0,2\left(mol\right)\\n_{Fe\left(OH\right)_2}=n_{Fe}=0,3\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{kết.tủa}=m_{Fe\left(OH\right)_3}+m_{Mg\left(OH\right)_2}=0,3\cdot107+0,2\cdot56=43,3\left(g\right)\)
Theo các PTHH: \(n_{H_2SO_4\left(p/ứ\right)}=0,5\left(mol\right)\) \(\Rightarrow n_{H_2SO_4\left(ban.đầu\right)}=0,5\cdot120\%=0,6\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,6\cdot98}{10\%}=588\left(g\right)\)
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{MgO}=n_{Mg}=0,2\left(mol\right)\\n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{chất.rắn}=m_{MgO}+m_{Fe_2O_3}=0,2\cdot40+0,15\cdot160=32\left(g\right)\)


a.
2 should
3 must
4 must / have to
5 don't have to
6 don't have to
7 should
8 mustn't
9 shouldn't
10 must / should
b.
2. You should have a rest.
3. Everyone will have to speak.
4. v
5. I had to stay in bed.
6. You mustn't park
7. v
8. People shouldn't answer