a. Tính khối lượng mol của H20 b. Tính phân tử khối của NO.
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) nCO2=[(9.1023)/(6.1023)]=1,5(mol)
=> mCO2=1,5.44=66(g)
V(CO2,đktc)=1,5.22,4=33,6(l)
b) nH2=4/2=2(mol)
N(H2)=2.6.1023=12.1023(phân tử)
V(H2,đktc)=2.22,4=44,8(l)
c) N(CO2)=0,5.6.1023=3.1023(phân tử)
V(CO2,đktc)=0,5.22,4=11,2(l)
mCO2=0,5.44=22(g)
d) nN2=2,24/22,4=0,1(mol)
mN2=0,1.28=2,8(g)
N(N2)=0,1.1023.6=6.1022 (phân tử)
e) nCu=[(3,01.1023)/(6,02.1023)]=0,5(mol)
mCu=0,5.64=32(g)
Mà sao tính thể tích ta :3

Câu 1
\(m_{HNO_3}=0,3.63=18,9\left(g\right)\)
\(m_{CuSO_4}=1,5.160=240\left(g\right)\)
\(m_{AlCl_3}=2.133,5=267\left(g\right)\)
Câu 2
a) \(V_{N_2}=3.22,4=67,2\left(l\right)\)
\(V_{H_2}=0,45.22,4=10,08\left(l\right)\)
\(V_{O_2}=0,55.22,4=12,32\left(l\right)\)
b) \(V_{hh}=\left(0,25+0,75\right).22,4=22,4\left(l\right)\)

mSO2= 0,2.(32+16.2)= 8,8(g)
nCl2= \(\frac{0,6.10^{23}}{6.10^{23}}\)=0,1 mol
mCl2= 0,1. 35,5.2 = 7,1(g)
nN2= \(\frac{1,2.10^{23}}{6.10^{23}}\)=0,2 mol
mN2= 0,2.14.2= 5,6 (g)
=> mA= 8,8+7,1+5,6=21,5 (g)

1)
a) \(n_{HCl}=\dfrac{7,3}{36,5}=0,2\left(mol\right)\)
b) \(n_{CH_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
c) \(n_{H_2O}=\dfrac{15.10^{23}}{6.10^{23}}=2,5\left(mol\right)\)
2)
a) \(n_A=\dfrac{2,24}{22,4}=0,1\left(mol\right)\) => MA = \(\dfrac{3}{0,1}=30\left(g/mol\right)\)
b) \(d_{A/O_2}=\dfrac{30}{32}=0,9375\)

Bài 7:
\(a.m_{Fe}=0,5.56=28\left(g\right)\\ b.n_{p.tử}=\dfrac{6.10^{23}}{6.10^{23}}=1\left(mol\right)\\ m_{CO_2}=44.1=44\left(g\right)\\ m_{Al_2O_3}=1.102=102\left(g\right)\\ m_{C_6H_{12}O_6}=180.1=180\left(g\right)\\ m_{H_2SO_4}=98.1=98\left(g\right)\)
Bài 8:
\(a.n_{Ca}=\dfrac{112}{40}=2,8\left(mol\right)\\ b.m_{HCl}=36,5.0,5=18,25\left(g\right)\\ c.n_{H_2SO_4}=\dfrac{49}{98}=0,5\left(mol\right)\)

a) \(m_{Na}=n.M=0,3.23=6,9\left(g\right)\)
\(m_{O_2}=n_{O_2}.M_{O_2}=0,3.32=9,6\left(g\right)\)
b) \(m_{HNO_3}=n_{HNO_3}.M_{HNO_3}=1,2.63=75,6\left(g\right)\)
\(m_{Cu}=n.M=0,5.64=32\left(g\right)\)
c) \(m_{KNO_3}=n.M=0,125=0,125.101=12,625\left(g\right)\)
\(m_{KMnO_4}=n.M=0,125.158=19,75\left(g\right)\)
\(m_{KClO_3}=n.M=0,125.122,5=15,3125\left(g\right)\)

\(a.n_{CO_2}=\dfrac{18.10^{23}}{6.10^{23}}=3\left(mol\right)\\ \Rightarrow m_{CO_2}=44.3=132\left(g\right)\\ b.n_{H_2O}=\dfrac{39,6}{18}=2,2\left(mol\right)\\ c.n_{Fe}=\dfrac{12.10^{23}}{6.10^{23}}=2\left(mol\right)\)

\(a_1,m_{CaCO_3}=0,25.100=25(g)\\ a_2,m_{SO_2}=\dfrac{3,36}{22,4}.64=9,6(g)\\ a_3,m_{H_2SO_4}=\dfrac{9.10^{23}}{6.10^{23}}.98=147(g)\)
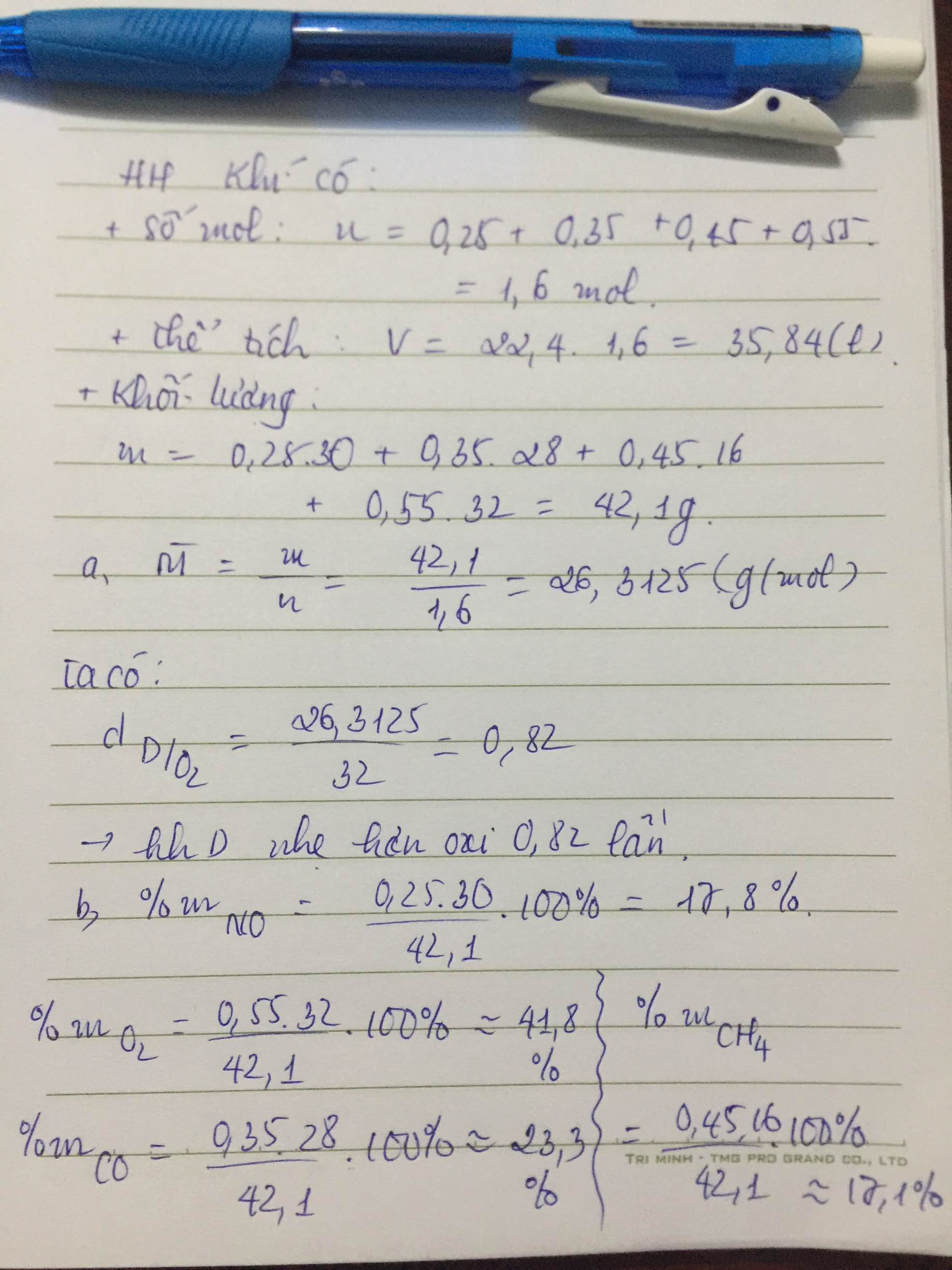
a) \(M_{H_2O}=1.2+16=18\left(\dfrac{g}{mol}\right)\)
b) \(M_{NO}=14+16=30\left(DvC\right)\)
Chữ đ trong đvC ko viết hoa