Trung hòa m gam dung dịch NaOH 20% cần dùng 192 gam dung dịch HCl 7,3% được dung dịch X.
Tính m? Tính C% của chất tan trong dung dịch X?
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(1,PTHH:4Al+3O_2\xrightarrow{t^o}2Al_2O_3\\ Al_2O_3+6HCl\to 2AlCl_3+3H_2\\ 2,n_{HCl}=\dfrac{240.7,3\%}{100\%.36,5}=0,48(mol)\\ \Rightarrow n_{Al_2O_3}=\dfrac{1}{6}n_{HCl}=0,08(mol)\\ \Rightarrow n_{Al}=2n_{Al_2O_3}=0,16(mol)\\ \Rightarrow m_{Al}=0,16.27=4,32(g)\\ n_{H_2}=\dfrac{1}{2}n_{HCl}=0,24(mol)\\ n_{AlCl_3}=\dfrac{1}{3}n_{HCl}=0,16(mol)\\ \Rightarrow C\%_{AlCl_3}=\dfrac{0,16.133,5}{0,08.102+240-0,24.2}.100\%=8,62\%\)

Giả sử số mol của HCl là 1 mol
PTHH: \(HCl+NaOH\rightarrow NaCl+H_2O\)
Theo PTHH: \(n_{NaCl}=n_{NaOH}=1mol=n_{HCl}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{36,5}{7,3\%}=500\left(g\right)\\m_{ddNaOH}=\dfrac{40}{20\%}=200\left(g\right)\\m_{NaCl}=1\cdot58,5=58,5\left(g\right)\end{matrix}\right.\)
\(\Rightarrow C\%_{NaCl}=\dfrac{58,5}{200+500}\cdot100\%\approx8,36\%\)
Vậy nồng độ phần trăm chất tan là 8,36%

Ta có: \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
a. PTHH: \(Mg+2HCl--->MgCl_2+H_2\)
Theo PT: \(n_{Mg}=n_{H_2}=0,2\left(mol\right)\)
=> \(m_{Mg}=0,2.24=4,8\left(g\right)\)
Theo PT: \(n_{HCl}=2.n_{Mg}=2.0,2=0,4\left(mol\right)\)
=> \(m_{HCl}=0,4.36.5=14,6\left(g\right)\)
=> \(C_{\%_{HCl}}=\dfrac{14,6}{200}.100\%=7,3\%\)
b. Ta có: \(m_{dd_{MgCl_2}}=4,8+200=204,8\left(g\right)\)
Theo PT: \(n_{MgCl_2}=n_{Mg}=0,2\left(mol\right)\)
=> \(m_{MgCl_2}=0,2.95=19\left(g\right)\)
=> \(C_{\%_{MgCl_2}}=\dfrac{19}{204,8}.100\%=9,28\%\)

nH2=\(\frac{6,72}{22,4}=0,3\)mol
PTHH
M+2HCl--> MCl2+H2
0,3mol<---------------0,3mol
=>MM=\(\frac{19,5}{0,3}=64\)
=> km loại là kẽm (Zn)
b) nNaOH=0,2.1=0,2 mol
PTHH
NaOH+HCl-->NaCl + H2O
0,2 mol--> 0,2 mol
---> thể tích HCl 1M đã dùng là V=\(\frac{0,2+0,3}{1}=0,5\)lít
=> CM(ZnCl2)=\(\frac{0,3}{0,5}=0,6M\)

\(n_{Fe}=\dfrac{19,6}{56}=0,35\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,35--> 0,7-----> 0,35--> 0,35
\(m_{dd.HCl}=\dfrac{0,7.36,5.100\%}{7,3\%}=350\left(g\right)\\ m_{dd}=19,6+350-0,35.2=368,9\left(g\right)\\ C\%_{FeCl_2}=\dfrac{127.0,35.100\%}{368,9}=12,05\%\)

a)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
_____0,1<---0,2<-------0,1<---0,1
=> mHCl = 0,2.36,5 = 7,3 (g)
=> \(m_{ddHCl}=\dfrac{7,3.100}{7,3}=100\left(g\right)\)
mdd sau pư = 0,1.24 + 100 - 0,1.2 = 102,2 (g)
\(C\%\left(MgCl_2\right)=\dfrac{0,1.95}{102,2}.100\%=9,2955\%\)
b)
CTHH: AaOb
PTHH: \(A_aO_b+2bHCl->aACl_{\dfrac{2b}{a}}+bH_2O\)
____________0,2------->\(\dfrac{0,1a}{b}\)
=> \(\dfrac{0,1a}{b}\left(M_A+35,5.\dfrac{2b}{a}\right)=13,5\)
=> \(M_A=\dfrac{64b}{a}=\dfrac{2b}{a}.32\)
Nếu \(\dfrac{2b}{a}=1\) => MA = 32 (L)
Nếu \(\dfrac{2b}{a}=2\) => MA = 64(Cu)

a) \(n_{KCl}=0,3.2=0,6\left(mol\right)\)
=> \(m_{KCl}=0,6.74,5=44,7\left(g\right)\)
b) \(m_{NaOH}=20.25\%=5\left(g\right)\)
c) \(S=\dfrac{m_{ct}}{m_{dd}}.100\)
=> \(53,6=\dfrac{m_{MgCl_2}}{100}.100\)
=> mMgCl2 = 53,6 (g)
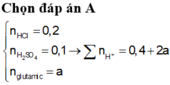
\(n_{HCl}=\dfrac{192.7,3}{100.36,5}=0,384\left(mol\right)\)
PTHH: NaOH + HCl --> NaCl + H2O
______0,384<-0,384->0,384____________(mol)
=> mNaOH = 0,384.40 = 15,36 (g)
=> \(m_{DD}=\dfrac{15,36.100}{20}=76,8\left(g\right)\)
\(C\%\left(NaCl\right)=\dfrac{0,384.58,5}{76,8+192}.100\%=8,36\%\)
\(n_{HCl}=\dfrac{192.7,3\%}{100\%.36,5}=0,384(mol)\\ PTHH:NaOH+HCl\to NaCl+H_2O\\ \Rightarrow n_{NaOH}=n_{HCl}=0,384(mol)\\ \Rightarrow m=m_{dd_{NaOH}}=\dfrac{0,384.40}{20\%}=76,8(g)\\ n_{NaCl}=n_{HCl}=0,384(mol)\\ \Rightarrow C\%_{NaCl}=\dfrac{0,384.58,5}{76,8+192}.100\%=8,36\%\)