Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
a, Giả sử: \(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_2}=y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow x+y=\dfrac{22,4}{22,4}=1\left(mol\right)\left(1\right)\)
Ta có: \(n_{CO_2}=\dfrac{35,84}{22,4}=1,6\left(mol\right)\)
Theo PT: \(\Sigma n_{CO_2}=n_{CH_4}+2n_{C_2H_2}\)
\(\Rightarrow x+2y=1,6\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,4\left(mol\right)\\y=0,6\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,4}{1}.100\%=40\%\\\text{ }\%V_{C_2H_2}=60\%\end{matrix}\right.\)
b, Theo PT: \(\Sigma n_{O_2}=2n_{CH_4}+\dfrac{5}{2}n_{C_2H_2}=2,3\left(mol\right)\)
\(\Rightarrow m_{O_2}=2,3.32=73,6\left(g\right)\)
c, PT: \(CO_2+2NaOH\rightarrow Na_2CO_3+H_2O\)
Theo PT: \(n_{Na_2CO_3}=n_{CO_2}=1,6\left(mol\right)\)
\(\Rightarrow C_{M_{Na_2CO_3}}=\dfrac{1,6}{0,8}=2M\)
Bạn tham khảo nhé!

a, Ta có \(n_{CH_4}+n_{C_2H_4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\left(1\right)\)
PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{CH_4}=0,05\left(mol\right)\\n_{C_2H_4}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{CH_4}=0,05.22,4=1,12\left(l\right)\\V_{C_2H_4}=0,1.22,4=2,24\left(l\right)\end{matrix}\right.\)
b, \(m_{CH_4}=0,05.16=0,8\left(g\right)\)
c, \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
Theo PT: \(n_{Br_2}=n_{C_2H_4}=0,1\left(mol\right)\Rightarrow V_{ddBr_2}=\dfrac{0,1}{1}=0,1\left(l\right)\)
\(m_{C_2H_4}=0,1.28=2,8\left(g\right)\)

\(n_{O_2}=\dfrac{8}{32}=0.25\left(mol\right)\)
\(CH_4+2O_2\underrightarrow{t^0}CO_2+2H_2O\)
\(0.125....0.25....0.125\)
\(m_{CH_4}=0.125\cdot16=2\left(g\right)\)
\(V_{CO_2}=0.125\cdot22.4=2.8\left(l\right)\)
\(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
\(............0.125.....0.125\)
\(m_{CaCO_3}=0.125\cdot100=12.5\left(g\right)\)

CH4+2O2-to>CO2+2H2O
x-----------------------------2x
C2H2+\(\dfrac{5}{2}\)O2-to>2CO2+H2O
y-----------------------------------y
=>\(\left\{{}\begin{matrix}x+y=\dfrac{3,36}{22,4}\\2x+y=\dfrac{4,5}{18}\end{matrix}\right.\)
=>\(\left\{{}\begin{matrix}x=0,1\\y=0,05\end{matrix}\right.\)
=>%VCH4=\(\dfrac{0,1.22,4}{3,36}\).100=66,67%
=>%VC2H2=100-66,67%=33,33%
b)
C2H2+2Br2->C2H2Br4
0,05-----0,1 mol
=>m Br2=0,1.160=16g

- Gọi mol metan và etan là x, y ( mol )
\(x+y=n_{hh}=\dfrac{V}{22,4}=0,25\left(mol\right)\)
Lại có : \(x+2y=n_{CO_2}=\dfrac{V}{22,4}=0,4\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,15\end{matrix}\right.\) ( mol )
\(\Rightarrow\left\{{}\begin{matrix}m_{CH_4}=1,6\left(g\right)\\m_{C_2H_6}=4,5\left(g\right)\end{matrix}\right.\)
=> mhh = 6,1 ( g )
=> %mCH4 = ~ 26,22%
=> %mC2H6 = ~73,78%
Ta có : \(\%V_{CH4}=\dfrac{V}{Vhh}=40\%\)
=> %VC2H6 = 100 - %VCH4 = 60% .
PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(2C_2H_6+5O_2\underrightarrow{t^o}4CO_2+6H_2O\)
Giả sử: \(\left\{{}\begin{matrix}n_{CH_4}=x\left(mol\right)\\n_{C_2H_6}=y\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow x+y=\dfrac{5,6}{22,4}=0,25\left(1\right)\)
Ta có: \(n_{CO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
Theo PT: \(\Sigma n_{CO_2}=n_{CH_4}+2n_{C_2H_6}\)
\(\Rightarrow x+2y=0,4\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,1\left(mol\right)\\y=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,1}{0,25}.100\%=40\%\\\%V_{C_2H_6}=60\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,1.16}{0,1.16+0,15.30}.100\%\approx26,2\%\\\%m_{C_2H_6}\approx73,8\%\end{matrix}\right.\)
Bạn tham khảo nhé!

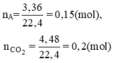
Gọi số mol của metan và etan lần lượt là x và y (mol)
Phương trình phản ứng:
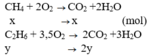
Vậy thành phần phần trăm về thể tích các khí trong hỗn hợp A là:
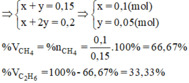

a)
\(m_{C_2H_2} = m_{tăng} = 5,2\ gam\\ \Rightarrow n_{C_2H_2} = \dfrac{5,2}{26} = 0,2(mol)\)
Vậy :
\(\%V_{C_2H_2} = \dfrac{0,2.22,4}{8,96}.100\% = 50\%\\ \%V_{CH_4} = 100\%-50\% = 50\%\)
b)
\(n_{CH_4} = n_{C_2H_2} = 0,2(mol)\)
CH4 + O2 \(\xrightarrow{t^o}\) CO2 + H2O
0,2.........................0,2...................................(mol)
C2H2 + \(\dfrac{5}{2}\)O2 \(\xrightarrow{t^o}\) 2CO2 + H2O
0,2................................0,4.................................(mol)
CO2 + Ca(OH)2 → CaCO3 + H2O
(0,2+0,4)............................(0,2+0,4)........................................(mol)
\(\Rightarrow m_{CaCO_3} =(0,2 + 0,4).100 = 60(gam)\)
\(Đặt:n_{C_2H_2}=a\left(mol\right),n_{CH_4}=b\left(mol\right)\)
\(n_{hh}=a+b=0.15\left(mol\right)\left(1\right)\)
\(C_2H_2\rightarrow2CO_2\)
\(CH_4\rightarrow CO_2\)
\(n_{CO_2}=2a+b=0.2\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.05,b=0.1\)
\(\%C_2H_2=\dfrac{0.05}{0.15}\cdot100\%=33.33\%\)
\(\%CH_4=66.67\%\)
\(2NaOH+CO_2\rightarrow Na_{_{ }2}CO_3+H_2O\)
\(0.4...............0.2............0.2\)
\(C_{M_{Na_2CO_3}}=\dfrac{0.2}{0.5}=0.4\left(M\right)\)
\(C_{M_{NaOH\left(dư\right)}}=\dfrac{0.5-0.4}{0.5}=0.2\left(M\right)\)