Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đáp án A
Thành phần % khối lượng của O = 100 – (52,17 + 13,04) = 34,79%
nC : nH : nO = 52,17/12 : 13,04 : 34,79/16 = 4,35 : 13,04 : 2,17 = 2: 6: 1
=> Công thức đơn giản nhất là C 2 H 6 O 2 .
M = (2x12+6+16)n = 46 => n =1
Vậy công thức phân tử: C 2 H 6 O 2

a)
\(m_C=\dfrac{52,15.46}{100}=24\left(g\right)=>n_C=\dfrac{24}{12}=2\left(mol\right)\)
\(m_H=\dfrac{13,04.46}{100}=6\left(g\right)=>n_H=\dfrac{6}{1}=6\left(mol\right)\)
\(m_O=46-24-6=16\left(g\right)=>n_O=\dfrac{16}{16}=1\left(mol\right)\)
=> CTHH: C2H6O
b) \(n_A=\dfrac{18,4}{46}=0,4\left(mol\right)\)
mC = 12.0,4.2 = 9,6(g)
mH = 1.0,4.6 = 2,4 (g)
mO = 16.0,4.1 = 6,4 (g)
c) \(n_A=\dfrac{13,8}{46}=0,3\left(mol\right)\)
Số nguyên tử C = 2.0,3.6.1023 = 3,6.1023
Số nguyên tử H = 6.0,3.6.1023 = 10,8.1023
Số nguyên tử O = 1.0,3.6.1023 = 1,8.1023

\(d_{\dfrac{X}{H_2}}=89\\ M_{H_2}=2\left(\dfrac{g}{mol}\right)\\ \Rightarrow M_X=d_{\dfrac{X}{H_2}}.M_{H_2}=89.2=178\left(\dfrac{g}{mol}\right)\)
\(m_C=\%C.M_X=74,16\%.178=132\left(g\right)\\ m_H=\%H.M_X=7,86\%.178=14\left(g\right)\\ m_O=m_X-m_C-m_H=178-132-14=32\left(g\right)\\\)
\(\Rightarrow n_C=\dfrac{m}{M}=\dfrac{132}{12}=11\left(mol\right)\\ n_H=\dfrac{m}{M}=\dfrac{14}{1}=14\left(mol\right)\\ n_O=\dfrac{m}{M}=\dfrac{32}{16}=2\left(mol\right)\)
\(CTHH:C_{11}H_{14}O_2\Rightarrow D\)

a) Xét mC : mH : mO = 64,865% : 13,51% : 21,625%
=> nC : nH : nO = \(\dfrac{64,865}{12}:\dfrac{13,51}{1}:\dfrac{21,625}{16}=4:10:1\)
=> CTPT: (C4H10O)n hay C4nH10nOn ( n thuộc N*)
Xét độ bất bão hòa \(=\dfrac{2.4n+2-10n}{2}=\dfrac{2-2n}{2}=1-n\ge0\)
=> n = 1
Vậy CTPT: C4H10O
CTCT:
(1) \(CH_3-CH_2-CH_2-CH_2OH\)
(2) \(CH_3-CH_2-CH\left(OH\right)-CH_3\)
(3) \(CH_3-CH\left(CH_3\right)-CH_2OH\)
(4) \(CH_3-C\left(OH\right)\left(CH_3\right)-CH_3\)
(5) \(CH_3-CH_2-CH_2-O-CH_3\)
(6) \(CH_3-CH\left(CH_3\right)-O-CH_3\)
(7) \(CH_3-CH_2-O-CH_2-CH_3\)
b) Xét mC : mH : mN = 61,017% : 15,254% : 23,729%
=> \(n_C:n_H:n_N=\dfrac{61,017}{12}:\dfrac{15,254}{1}:\dfrac{23,729}{14}=3:9:1\)
=> CTPT: (C3H9N)n hay C3nH9nNn ( n thuộc N*)
Xét độ bất bão hòa \(\dfrac{2.3n+2-9n+n}{2}=1-n\)
=> n = 1
=> CTPT: C3H9N
CTCT:
(1) \(CH_3-CH_2-CH_2-NH_2\)
(2) \(CH_3-CH\left(NH_2\right)-CH_3\)
(3) \(CH_3-CH_2-NH-CH_3\)
(4) \(\left(CH_3\right)N\)
a)
\(CTTQ:C_aH_bO_z\left(a,b,z:nguyên,dương\right)\\ Ta.có:a:b:z=\dfrac{64,865\%}{12}:\dfrac{13,51\%}{1}:\dfrac{100\%-\left(64,865\%+13,51\%\right)}{16}\\ =0,054:0,1351:0,0135=4:10:1\\ \Rightarrow a=4;b=10;z=1\\ \Rightarrow CTPT:C_4H_{10}O\\ CTCT:CH_3-CH_2-CH_2-CH_2-OH\left(1\right)\\ CH_3-CH\left(CH_3\right)-CH_2-OH\left(2\right)\\ CH_3-CH_2-CH\left(CH_3\right)-OH\left(3\right)\\ CH_3-C\left(OH\right)-CH\left(CH_3\right)-CH_3\left(4\right)\\ CH_3-CH_2-CH_2-O-CH_3\left(5\right)\\ CH_3-CH\left(CH_3\right)-O-CH_3\left(6\right)\\ CH_3-CH_2-O-CH_2-CH_3\left(7\right)\)
Gọi tên:
(1) Ancol butylic
(2) 2 - metylpropan - 1 - ol
(3) Butan - 2 - ol
(4) 2 - metylpropan - 2 - ol
(5) metylpropyl ete
(6) Isopropylmetyl ete
(7) Đietyl ete

\(m_C=\dfrac{52,17.46}{100}=24\left(g\right)=>n_C=\dfrac{24}{12}=2\left(mol\right)\)
\(m_H=\dfrac{13,04.46}{100}=6\left(g\right)=>n_H=\dfrac{6}{1}=6\left(mol\right)\)
\(m_O=46-24-6=16\left(g\right)=>n_O=\dfrac{16}{16}=1\left(mol\right)\)
=> CTHH: C2H6O
Đặt \(CTPT:C_xH_yO_z\)
\(\%_O=100\%-52,17\%-13,04\%=34,79\%\\ \Rightarrow x:y:z=\dfrac{52,17}{12}:\dfrac{13,04}{1}:\dfrac{34,79}{16}=2:6:1\\ \Rightarrow CTPT:(C_2H_6O)_n\\ \Rightarrow (12.2+6+16)n=46\\ \Rightarrow n=1\\ \Rightarrow CTPT:C_2H_6O\)

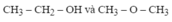
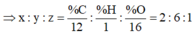
\(CT:C_xH_yO_z\)
\(\%O=100-52.17-13.04=34.79\%\)
\(x:y:z=\dfrac{52.17}{12}:\dfrac{13.04}{1}:\dfrac{34.79}{16}=4.3475:13.04:2.174375=2:6:1\)
\(CTnguyên:\left(C_2H_6O\right)_n\)
\(M_A=46\Leftrightarrow46n=46\Leftrightarrow n=1\)
\(CT:C_2H_6O\)