1) \(Al\xrightarrow[]{\left(1\right)}Al_2O_3\xrightarrow[]{\left(2\right)}AlCl_3\xrightarrow[]{\left(3\right)}Al\left(OH\right)_3\)
2) \(Al\xrightarrow[]{\left(1\right)}AlCl_3\xrightarrow[]{\left(2\right)}Al\left(OH\right)_3\xrightarrow[]{\left(3\right)}Al_2O_3\)
3) \(Fe\xrightarrow[]{\left(1\right)}FeSO_4\xrightarrow[]{\left(2\right)}FeCl_2\xrightarrow[]{\left(3\right)}Fe\left(OH\right)_2\xrightarrow[]{\left(4\right)}FeO\)
4) \(Fe\left(OH\right)_2\xrightarrow[]{\left(1\right)}FeO\xrightarrow[]{\left(2\right)}FeSO_4\xrightarrow[]{\left(3\right)}FeCl_2\xrightarrow[]{\left(4\right)}Fe\left(OH\right)_2\)
5) \(Fe\xrightarrow[]{\left(1\right)}FeCl_2\xrightarrow[]{\left(2\right)}Fe\left(NO_3\right)_2\xrightarrow[]{\left(3\right)}Fe\left(OH\right)_2\xrightarrow[]{\left(4\right)}FeSO_4\)
6) \(Fe\xrightarrow[]{\left(1\right)}FeCl_3\xrightarrow[]{\left(2\right)}Fe\left(OH\right)_3\xrightarrow[]{\left(3\right)}Fe_2\left(SO_4\right)_3\xrightarrow[]{\left(4\right)}FeCl_3\)
7) \(Fe\left(NO_3\right)_3\xrightarrow[]{\left(1\right)}Fe\left(OH\right)_3\xrightarrow[]{\left(2\right)}Fe_2O_3\xrightarrow[]{\left(3\right)}Fe\xrightarrow[]{\left(4\right)}FeCl_3\)
8) \(Fe_2\left(SO_4\right)_3\xrightarrow[]{\left(1\right)}Fe\left(OH\right)_3\xrightarrow[]{\left(2\right)}Fe_2O_3\xrightarrow[]{\left(3\right)}Fe_2\left(SO_4\right)_3\xrightarrow[]{\left(4\right)}FeCl_3\)

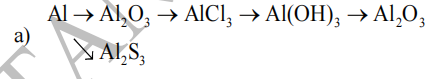
1
1)4 Al+3O2→2Al2O3
(2)Al2O3+6HCl→2AlCl3+3H2O
(3)AlCl3+3NaOH→Al(OH)3+3NaCl
2
4Al+3O2→2Al2O3
Al2O3+6HCl→2AlCl3+3H2O
AlCl3+3NaOH→Al(OH)3+3NaCl
3
Fe+H2SO4→FeSO4+H2
(2)FeSO4+BaCl2→FeCl2+BaSO4
(3)FeCl2+2NaOH→Fe(OH)2+2NaCl
(4)Fe(OH)2→FeO+H2O
4
Fe+H2SO4→FeSO4+H2
FeSO4+BaCl2→FeCl2+BaSO4
FeCl2+2NaOH→Fe(OH)2+2NaCl
Fe(OH)2→FeO+H2O
5
Fe+2HCl→FeCl2+H2
(2)FeCl2+2AgNO3→Fe(NO3)2+2AgCl
(3)Fe(NO3)2+2NaOH→Fe(OH)2+2NaNO3
(4)Fe(OH)2+MgSO4→FeSO4+Mg(OH)2
Câu 1 :
( 1 ) 4Al + 3O2 → 2Al2O3 ( Nhiệt độ )
( 2 ) Al2O3 + 6HCl → 2AlCl3 + 3H2O
( 3 ) AlCl3 + 3NaOH → Al(OH)3 + 3NaCl
Câu 2 :
( 1 ) 2Al + 6HCl → 2AlCl3 + 3H2
( 2 ) AlCl3 + 3NaOH → Al(OH)3 + 3NaCl
( 3 ) 2Al(OH)3 → Al2O3 + 3H2O ( Nhiệt độ )
Câu 3 :
( 1 ) Fe + H2SO4 → H2 + FeSO4
( 2 ) BaCl2 + FeSO4 → FeCl2 + BaSO4
( 3 ) FeCl2 + 2NaOH → NaCl + Fe(OH)2
( 4 ) Fe(OH)2 → FeO + H2O ( Nhiệt độ )
Câu 4 :
( 1 ) Fe(OH)2 → FeO + H2O ( Nhiệt độ )
( 2 ) FeO + H2SO4 → H2O + FeSO4
( 3 ) BaCl2 + FeSO4 → FeCl2 + BaSO4
( 4 ) FeCl2 + 2NaOH → NaCl + Fe(OH)2
Câu 5 :
( 1 ) Fe + 2HCl → FeCl2 + H2
( 2 ) 2AgNO3 + FeCl2 → 2AgCl + Fe(NO3)2
( 3 ) Fe(NO3)2 + NaOH → NaNO3 + Fe(OH)2
( 4 ) H2SO4 + Fe(OH)2 → 2H2O + FeSO4
Câu 6 :
( 1 ) 3Cl2 + 2Fe → 2FeCl3 ( Nhiệt độ )
( 2 ) 3NaOH + FeCl3 → 3NaCl + Fe(OH)3
( 3 ) 3H2SO4 + 2Fe(OH)3 → Fe2(SO4)3 + 6H2O
( 4 ) 3BaCl2 + Fe2(SO4)3 → 2FeCl3 + 3BaSO4
Câu 7 :
( 1 ) 3NaOH + Fe(NO3)3 → 3NaNO3 + Fe(OH)3
( 2 ) 2Fe(OH)3 → Fe2O3 + 3H2O ( Nhiệt độ )
( 3 ) 2Al + Fe2O3 → Al2O3 + 2Fe
( 4 ) 3Cl2 + 2Fe → 2FeCl3 ( Nhiệt độ )
Câu 8 :
( 1 ) Fe2(SO4)3 + 6NaOH → 3Na2SO4 + 2Fe(OH)3
( 2 ) 2Fe(OH)3 → Fe2O3 + 3H2O ( Nhiệt độ )
( 3 ) Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
( 4 ) 3BaCl2 + Fe2(SO4)3 → 2FeCl3 + 3BaSO4