Tính [ion] các chất có trong dung dịch sau đây:
a. Dd Cu(NO3)2 0,3 M.
b. Hòa tan 4,9g H2SO4 vào nước thu được 200 ml dung dịch.
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) Ta có: \(n_{NaCl}=\dfrac{5,85}{58,5}=0,1\left(mol\right)\)
\(\Rightarrow C_{M_{NaCl}}=\dfrac{0,1}{0,5}=0,2\left(M\right)=\left[Na^+\right]=\left[Cl^-\right]\)
b) Ta có: \(n_{Ba\left(OH\right)_2}=\dfrac{34,2}{171}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{Ba\left(OH\right)_2}}=\dfrac{0,2}{0,5}=0,4\left(M\right)\) \(\Rightarrow\left\{{}\begin{matrix}\left[Ba^{2+}\right]=0,4\left(M\right)\\\left[OH^-\right]=0,8\left(M\right)\end{matrix}\right.\)
c) Ta có: \(n_{H_2SO_4}=0,025\cdot2=0,05\left(mol\right)\)
\(\Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,05}{0,125+0,025}\approx0,33\left(M\right)\) \(\Rightarrow\left\{{}\begin{matrix}\left[H^+\right]=0,66\left(M\right)\\\left[SO_4^{2-}\right]=0,33\left(M\right)\end{matrix}\right.\)

Khj cho B td H2SO4 ko co chat khj thoat ra chung to Al va Zn da pu het.
nCu(NO3)2=0,03=>nCu[+2]=0,03.
nAgNO3=0,01=>nAg+=0,01
goi x,y la so mol Al,Zn.
Al>Al[+3]+3e
Zn>Zn[+2]+2e
=>ne nhuog=3x+2y
Cu[+2]+2e>Cu
Ag+ + 1e>Ag
=>ne nhan=0,03.2+0,01=0,07
theo dlbt e=>3x+2y=0,07
27x+65y=1,57
=>x=0,01,y=0,02
=>nAl(NO3)3=0,01
=>mAl(NO3)3=2,13g
nZn(NO3)2=nZn[+2]=0,02=>mZn(NO3)2=3,78g
khoi luog Cu va Ag la=0,03.64+0,01.108=3g
=>kl dd giam la 3-1,57=1,43
=>kl dd luc sau la 101,43-1,43=100g
=>C%Al(NO3)3=2,13/100=2,13%
C%Zn(NO3)2=3,78%

a)
Gọi $n_{CuO} = a(mol) ; n_{Mg} = b(mol)$
$CuO + 2HNO_3 \to Cu(NO_3)_2 + H_2O$
$3Mg + 8HNO_3 \to 3Mg(NO_3)_2 + 2NO + 4H_2O$
Theo PTHH :
$n_{HNO_3} = 2a + \dfrac{8}{3}b = 0,2.3 = 0,6(mol)$
$n_{NO} = \dfrac{2}{3}b = \dfrac{1,12}{22,4} = 0,05(mol)$
Suy ra a = 0,2 ; b = 0,075
$m = 0,2.80 + 0,075.24 = 17,8(gam)$
b)
$C_{M_{Cu(NO_3)_2}} = \dfrac{0,2}{0,2} = 1M$
$C_{M_{Mg(NO_3)_2}} = \dfrac{0,075}{0,2} = 0,375M$

a, \(\left[Ca^{2+}\right]=\dfrac{0,15.0,5}{0,15+0,05}=0,375M\)
\(\left[Na^+\right]=\dfrac{0,05.2}{0,15+0,05}=0,5M\)
\(\left[Cl^-\right]=\dfrac{0,15.2.0,5+0,05.2}{0,15+0,05}=1,25M\)

\(a,m_{rắn}=m_{Cu}=2,7\left(g\right)\\ \Rightarrow m_{\left(Zn,Fe\right)}=12-2,7=9,3\left(g\right)\\ n_{H_2}=0,15\left(mol\right),n_{axit}=2.0,2=0,4\left(mol\right)\\ Đặt:n_{Zn}=a\left(mol\right);n_{Fe}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ Vì:\dfrac{0,15}{1}< \dfrac{0,4}{1}\Rightarrow axit.dư\\ \Rightarrow\left\{{}\begin{matrix}65+56b=9,3\\a+b=\dfrac{3,36}{22,4}=0,15\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,05\end{matrix}\right.\\ \Rightarrow\%m_{Cu}=\dfrac{2,7}{12}.100=22,5\%\\ \%m_{Zn}=\dfrac{0,1.65}{12}.100\approx54,167\%\\ \%m_{Fe}=\dfrac{0,05.56}{12}.100\approx23,333\%\)
\(b,ddA:FeCl_2,ZnCl_2,H_2SO_4\left(dư\right)\\ m_{ddH_2SO_4}=200.1,14=228\left(g\right)\\ m_{ddA}=m_{\left(Zn,Fe\right)}+m_{ddH_2SO_4}-m_{H_2}=9,3+228-0,15.2=237\left(g\right)\)
\(C\%_{ddZnCl_2}=\dfrac{136.0,1}{237}.100\approx5,738\%\\ C\%_{ddFeCl_2}=\dfrac{127.0,05}{237}.100\approx2,679\%\\ C\%_{ddH_2SO_4\left(dư\right)}=\dfrac{\left(0,4-0,15\right).98}{237}.100\approx10,338\%\)
Đã sửa lần cuối lúc 20:45

Đáp án B
nCu = nCuO = x; nCu(NO3)2 = y
Dung dịch X chỉ chứa 1 chất tan (CuSO4) => Ion NO3- đã hết
3Cu + 8H+ + 2NO3-→ 3Cu2+ + 2NO + 4H2O
3y ← 8y ← 2y
O2- + 2H+→ H2O
x → 2x
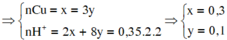
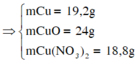
=> %mCu = 30,968% => Chọn B.
a) Ta có: \(\left\{{}\begin{matrix}\left[Cu^{2+}\right]=C_{M_{Cu\left(NO_3\right)_2}}=0,3\left(M\right)\\\left[NO_3^-\right]=2C_{M_{Cu\left(NO_3\right)_2}}=0,6\left(M\right)\end{matrix}\right.\)
b) Ta có: \(n_{H_2SO_4}=\dfrac{4,9}{98}=0,05\left(mol\right)\) \(\Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,05}{0,2}=0,25\left(M\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\left[H^+\right]=0,5\left(M\right)\\\left[SO_4^{2-}\right]=0,25\left(M\right)\end{matrix}\right.\)