FeClx + Cl2 -> FeCl3
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a. NaCl → Cl2 → FeCl3 → NaCl → HCl → CuCl2 → AgCl.
2NaCl+2H2O-đp\mn->2NaOH+H2+Cl2
3Cl2+2Fe-to>2FeCl3
FeCl3+3NaOH->3NaCl+Fe(OH)3
2NaCl+H2SO4-to>Na2SO4+2HCl
2HCl+CuO->CuCl2+H2O
CuCl2+2AgNO3->2AgCl+Cu(NO3)2

1,
\(4HCl+MnO_2\rightarrow MnCl_2+2H_2O+Cl_2\\ 2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3\\ FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\\ 2NaCl+H_2SO_4\rightarrow Na_2SO_4+2HCl\uparrow\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ CuCl_2+2AgNO_3\rightarrow2AgCl\downarrow+Cu\left(NO_3\right)_2\)
2,
\(2KMnO_4+16HCl\rightarrow2KCl+8H_2O+5Cl_2+2MnCl_2\\ Cl_2+H_2\underrightarrow{as}2HCl\\ 6HCl+Fe_2O_3\rightarrow2FeCl_3+3H_2O\\ FeCl_3+3AgNO_3\rightarrow3AgCl\downarrow+Fe\left(NO_3\right)_3\\ 2AgCl\underrightarrow{as}2Ag+Cl_2\\ Cl_2+2NaBr\rightarrow2NaCl+Br_2\\ Br_2+2NaI\rightarrow2NaBr+I_2\)
3,
2 pthh đầu giống ở 2
\(Fe+2HCl\rightarrow FeCl_2+H_2\\ FeCl_2+2AgNO_3\rightarrow2AgCl\downarrow+Fe\left(NO_3\right)_2\\ 2AgCl\underrightarrow{as}2Ag+Cl_2\)
4, 2 pthh đầu gióng ở 1
\(FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\\ 2Fe\left(OH\right)_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+6H_2O\)

$a) MnO_2 + 4HCl \xrightarrow{t^o} MnCl_2 + Cl_2 + 2H_2O$
$Cl_2 + Cu \xrightarrow{t^o} CuCl_2$
$CuCl_2 + 2NaOH \to Cu(OH)_2 + 2NaCl$
$2NaCl + 2H_2O \xrightarrow{đpdd, cmn} 2NaOH + H_2 + Cl_2$
$2NaOH + Cl_2 \to NaCl + NaClO + H_2O$
$NaClO + HCl \to NaCl + HClO$
b)
$2KMnO_4 + 16HCl \to 2KCl + 2MnCl_2 + 5Cl_2 + 8H_2O$
$Cl_2 + H_2 \xrightarrow{ánh\ sáng} 2HCl$
$HCl + KOH \to KCl + H_2O$
$2KCl + 2H_2O \xrightarrow{đpdd, cmn} 2KOH + H_2 + Cl_2$
$3Cl_2 + 2Fe \xrightarrow{t^o} 2FeCl_3$
$FeCl_3 + 3AgNO_3 \to Fe(NO_3)_3 + 3AgCl$

a)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3\)
\(FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\)
\(2NaCl+H_2SO_4\underrightarrow{t^o}Na_2SO_4+2HCl\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\downarrow\)
b)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(H_2+Cl_2\underrightarrow{t^o}2HCl\)
\(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\)
\(FeCl_3+3AgNO_3\rightarrow Fe\left(NO_3\right)_3+3AgCl\downarrow\)
\(2AgCl\underrightarrow{as}2Ag+Cl_2\)
\(2NaBr+Cl_2\rightarrow2NaCl+Br_2\)
\(2NaI+Br_2\rightarrow2NaBr+I_2\)
\(Zn+I_2\underrightarrow{H_2O}ZnI_2\)
\(ZnI_2+2NaOH\rightarrow2NaI+Zn\left(OH\right)_2\)
c)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(3Cl_2+6KOH\underrightarrow{t^o}5KCl+KClO_3+3H_2O\)
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(2KCl+H_2SO_4\underrightarrow{t^o}K_2SO_4+2HCl\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(Ca\left(OH\right)_2+Cl_2\rightarrow CaOCl_2+H_2O\)

a)
\(4HCl+MnO_2\rightarrow MnCl_2+Cl_2+2H_2O\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
\(FeCl_3+3NaOH\rightarrow3NaCl+Fe\left(OH\right)_3\)
\(NaCl+AgNO_3\rightarrow NaNO_3+AgCl\)
b)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl2+5Cl_2+8H_2O\)
\(Cl_2+H_2\rightarrow2HCl\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2FeCl_2+Cl_2\rightarrow2FeCl_3\)
\(FeCl_3+3AgNO_3\rightarrow Fe\left(NO_3\right)_3+3AgCl\)
\(2AgCl\rightarrow2Ag+Cl_2\)
\(Cl_2+2NaBr\rightarrow2NaCl+Br_2\)

\(H_2 + Cl_2 \xrightarrow{ánh\ sáng} 2HCl\\ Fe + 2HCl \to FeCl_2 + H_2\\ 2FeCl_2 + Cl_2 \to 2FeCl_3\\ FeCl_3 + 3KOH \to Fe(OH)_3 + 3KCl\\ Fe(OH)_3 + 3HCl \to FeCl_3 + 3H_2O\\ 4HCl + MnO_2 \to MnCl_2 + Cl_2 + 2H_2O\\ \)
\(2NaOH + Cl_2 \to NaCl + NaClO + H_2O\\ NaCl + H_2SO_4 \xrightarrow{t^o} NaHSO_4 + HCl\\ CuO + 2HCl \to CuCl_2 + H_2O\\ CuCl_2 + 2AgNO_3 \to Cu(NO_3)_2 + 2AgCl\)

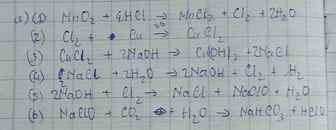
xFeClx+Cl2\(\rightarrow\) xFeCl3