Hòa tan m(g) hỗn hợp CuO , Al2O3 bằng 1 lượng vừa đủ 800ml Hcl 0,1 M thu được 4,02(g) muối .Tính C% mỗi chất trong hỗn hợp ban đầu
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Đặt \(\hept{\begin{cases}x=n_{CuO}\\y=n_{Al_2O_3}\end{cases}}\)
PTHH: \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
Theo phương trình \(\hept{\begin{cases}x=n_{CuCl_2}\\2y=n_{AlCl_3}\end{cases}}\)
\(\rightarrow135x+133,5.2y=4,02\left(1\right)\)
\(n_{HCl}=0,8.0,1=0,08mol\)
\(\rightarrow2x+6y=0,08\left(2\right)\)
Từ (1) và (2) => x = y = 0,01 mol
\(\rightarrow\%m_{CuO}=\frac{0,01.80}{0,01.80+0,01.102}.100\%=43,96\%\)
\(\rightarrow\%m_{Al_2O_3}=100-43,96\%=56,04\%\)
Đặt:\(\hept{\begin{cases}x=n_{CuO}\\y=n_{al_2O_3}\end{cases}}\)
PTHH:\(CuO+2HCL\rightarrow CuCl_2+H_2O\)
\(Al_2O_3+6HCL\rightarrow2Alcl_3+3H_2O\)
Theo phương trình: \(\hept{\begin{cases}x=n_{CuCl_2}\\2y=n_{AlCl_3}\end{cases}}\)
\(\rightarrow135x+133,5\cdot2y=4,02\left(1\right)\)
\(n_{HCL}=0,8\cdot0,1=0,08mol\)
\(\rightarrow2x+6y=0,08\left(2\right)\)
\(\Rightarrow x=y=0,01mol\)
\(\rightarrow\%m_{CuO}=\frac{0,01\cdot80}{0,01\cdot80+0,01\cdot102}\cdot100=43,96\%\)
\(\rightarrow\%m_{al_2O_3}=100\%-43,96\%=56,04\%\)

Gọi \(\left\{{}\begin{matrix}n_{CuO}=x\\n_{MgO}=y\end{matrix}\right.\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
x x ( mol )
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}80x+40y=16\\135x+95y=32,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{CuO}=0,1.80=8g\)
\(\Rightarrow m_{MgO}=0,2.40=8g\)
\(\%m_{CuO}=\dfrac{8}{16}.100=50\%\)
\(\%m_{MgO}=\dfrac{8}{16}.100=50\%\)
\(m_{CuCl_2}=0,1.135=13,5g\)
\(m_{MgCl_2}=0,2.95=19g\)

\(n_{H2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,2 0,4 0,2 0,2
\(Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O|\)
1 6 2 3
0,2 1,2 0,4
\(n_{Fe}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
⇒ \(m_{Fe}=0,2.56=11,2\left(g\right)\)
\(m_{Fe2O3}=27,2-11,2=16\left(g\right)\)
0/0Fe = \(\dfrac{11,2.100}{27,2}=41,18\)0/0
0/0Fe2O3 = \(\dfrac{16.100}{27,2}=58,82\)0/0
b) Có : \(m_{Fe2O3}=16\left(g\right)\)
\(n_{Fe2O3}=\dfrac{16}{160}=0,1\left(mol\right)\)
\(n_{HCl\left(tổng\right)}=0,4+1,2=1,6\left(mol\right)\)
\(V_{HCl}=\dfrac{1,6}{2}=0,8\left(l\right)\)
c) \(n_{FeCl2}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
\(n_{FeCl3}=\dfrac{1,2.2}{6}=0,4\left(mol\right)\)
\(C_{M_{FeCl2}}=\dfrac{0,2}{0,8}=0,25\left(M\right)\)
\(C_{M_{FeCl3}}=\dfrac{0,4}{0,8}=0,5\left(M\right)\)
Chúc bạn học tốt

Đặt :
nFe = x mol
nMgO = y mol
mX = 56x + 40y = 13.6 (g) (1)
Fe + 2HCl => FeCl2 + H2
x____________x
MgO + 2HCl => MgCl2 + H2O
y______________y
mM = mFeCl2 + mMgCl2 = 127x + 95y = 31.7 (2)
(1) , (2) :
x = 0.1
y = 0.2
%Fe = 5.6/13.6 * 100% = 41.17%
%MgO = 58.82%
nKOH = 0.1 * 0.2 = 0.02 (mol)
KOH + HCl => KCl + H2O
0.02____0.02
nHCl (pư) = 2nFe + 2nMgO = 0.1*2 + 0.2*2 = 0.6 (mol)
nHCl = 0.02 + 0.6 = 0.62 (mol)
VddHCl = 0.62/0.5 = 1.24 (M)

a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
b, Ta có: \(n_{H_2}=0,15\left(mol\right)\)
Theo PT: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al}=0,1.27=2,7\left(g\right)\)
\(\Rightarrow m_{Al_2O_3}=7,8-2,7=5,1\left(g\right)\)
c, Có: \(n_{Al_2O_3}=\dfrac{5,1}{102}=0,05\left(mol\right)\)
Theo PT: \(n_{HCl}=3n_{Al}+6n_{Al_2O_3}=0,6\left(mol\right)\)
\(n_{AlCl_3}=n_{Al}+2n_{Al_2O_3}=0,2\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,6.36,5=21,9\left(g\right)\Rightarrow m_{ddHCl}=\dfrac{21,9}{10\%}=219\left(g\right)\)
⇒ m dd sau pư = 7,8 + 219 - 0,15.2 = 226,5 (g)
\(\Rightarrow C\%_{AlCl_3}=\dfrac{0,2.133,5}{226,5}.100\%\approx11,79\%\)
Bạn tham khảo nhé!
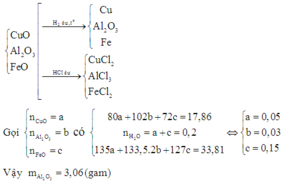


\(CuO+2HCl \to CuCl_2+H_2O\\ Al_2O_3+6HCl \to 2AlCl_3+3H_2O\\ n_{CuO}=a(mol)\\ n_{Al_2O_3}=b(mol)\\ n_{HCl}=2a+6b=0,08(1)\\ m_{muối}=135a+267b=4,02(2)\\ (1)(2)\\ a=b=0,01(mol)\\ m_{dd}=4,02+0,01.(80+102)=5,84g\\ C\%_{CuO}=\frac{0,01.80}{5,84}.100\%=13,7\%\\ C\%_{Al_2O_3}=\frac{0,01.102}{5,84}.100\%=17,4\%\)