Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:Fe+H_2SO_4\to FeSO_4+H_2\\ b,n_{H_2}=\dfrac{16,8}{22,4}=0,74(mol)\\ \Rightarrow n_{Fe}=0,75(mol)\\ \Rightarrow m_{Fe}=0,75.56=42(g)\\ c,n_{H_2SO_4}=\dfrac{245.10\%}{100\%.98}=0,25(mol)\)
Vì \(\dfrac{n_{Fe}}{1}>\dfrac{n_{H_2SO_4}}{1}\) nên \(Fe\) dư
\(n_{Fe(dư)}=0,75-0,25=0,5(mol)\\ \Rightarrow m_{Fe(dư)}=0,5.56=28(g)\)

a)
$Fe + 2HCl \to FeCl_2 + H_2$
Theo PTHH :
$n_{Fe} = n_{H_2} = \dfrac{11,2}{22,4} = 0,5(mol)$
$A = 0,5.56 = 28(gam)$
b) $n_{HCl} = 2n_{H_2} = 1(mol)$
$m_{HCl} = 1.36,5 = 36,5(gam)$
c) $m_{dd\ HCl} = 36,5 : 20\% = 182,5(gam)$
$m_{dd\ sau\ pư} = 28 + 182,5 - 0,5.2 = 209,5(gam)$
$C\%_{FeCl_2} = \dfrac{0,5.127}{209,5}.100\% = 30,3\%$

PT: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\) (1)
\(2Fe+6H_2SO_{4\left(đ\right)}\underrightarrow{t^o}Fe_2\left(SO_4\right)_3+3SO_2+6H_2O\) (2)
\(5SO_2+2KMnO_4+2H_2O\rightarrow2MnSO_4+K_2SO_4+2H_2SO_4\) (3)
Ta có: \(n_{H_2}=0,2\left(mol\right)\)
Theo PT (1): \(n_{Fe}=n_{H_2}=0,2\left(mol\right)\)
Theo PT (2): \(n_{SO_2}=\dfrac{3}{2}n_{Fe}=0,3\left(mol\right)\)
\(\Rightarrow V_{SO_2}=0,3.22,4=6,72\left(l\right)\)
Theo PT (3): \(n_{KMnO_4}=\dfrac{2}{5}n_{SO_2}=0,12\left(mol\right)\)
\(\Rightarrow V_{KMnO_4}=\dfrac{0,12}{2}=0,06\left(l\right)\)
Bạn tham khảo nhé!

\(n_{Fe}=\dfrac{12}{56}=\dfrac{3}{14}\left(mol\right)\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(\dfrac{3}{14}....\dfrac{3}{14}.......\dfrac{3}{14}......\dfrac{3}{14}\)
\(m_{FeSO_4}=\dfrac{3}{14}\cdot152=32.57\left(g\right)\)
\(V_{H_2}=\dfrac{3}{14}\cdot22.4=4.8\left(l\right)\)
\(m_{dd_{H_2SO_4}}=\dfrac{\dfrac{3}{14}\cdot98}{19.6\%}=107.1\left(g\right)\)

\(Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2\)
b)
\(n_{H_2}= \dfrac{2,24}{22,4}= 0,1 mol\)
\(\)Theo PTHH:
\(n_{ZnSO_4}= n_{H_2}= 0,1 mol\)
\(m_{ZnSO_4}= 0,1 . 161=16,1g\)
c)
Theo PTHH:
\(n_{H_2SO_4}= n_{H_2}= 0,1 mol\)
\(\Rightarrow m_{H_2SO_4}= 0,1 . 98= 9,8g\)
\(\Rightarrow m_{dd H_2SO_4}= \dfrac{9,8 . 100}{20}=49g\)
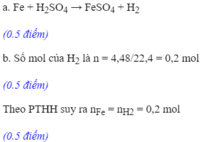

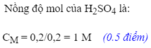
a) Fe + H2SO4 → FeSO4 + H2
b) Ta có : nH2 = \(\dfrac{16,8}{22,4}\) = 0,75 (mol)
⇒ nFe= 0,75.56 = 42(gam)
Ta có: \(n_{H_2}=\dfrac{16,8}{22,4}=0,75\left(mol\right)\)
a. \(PTHH:Fe+H_2SO_4--->FeSO_4+H_2\uparrow\)
b. Theo PT: \(n_{Fe}=n_{H_2}=0,75\left(mol\right)\)
\(\Rightarrow m_{Fe}=56.0,75=42\left(g\right)\)