Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)

`a)PTHH`
`Fe + 2HCl -> FeCl_2 + H_2`
`0,125` `0,25` `0,125` `0,125` `(mol)`
`n_[HCl]=[5/100 .182,5]/[36,5]=0,25(mol)`
`b)m_[Fe]=0,125.56=7(g)`
`V_[H_2]=0,125.22,4=2,8(l)`
`c)m_[HCl]=0,25.36,5=9,125(g)`
`m_[FeCl_2]=0,125.127=15,875(g)`
`d)C%_[FeCl_2]=[15,875]/[7+182,5-0,125.2] .100~~8,39%`

\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,1---->0,3
Zn + H2SO4 --> ZnSO4 + H2
0,3<--------------------0,3
=> m = 0,3.65 = 19,5 (g)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow m_{ZnCl_2}=0,2.136=27,2\left(g\right)\)
d, \(n_{HCl}=2n_{Zn}=0,4\left(mol\right)\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{14,6}{7,3\%}=200\left(g\right)\)
⇒ m dd sau pư = 13 + 200 - 0,2.2 = 212,6 (g)
\(\Rightarrow C\%_{ZnCl_2}=\dfrac{27,2}{212,6}.100\%\approx12,79\%\)

a.b.
\(n_{Na}=\dfrac{13,8}{23}=0,6mol\)
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,6 0,3 ( mol )
\(V_{H_2}=0,3.22,4=6,72l\)
c.\(n_{CuO}=\dfrac{12}{80}=0,15mol\)
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,15 < 0,3 ( mol )
0,15 0,15 ( mol )
Chất dư là H2
\(m_{H_2}=\left(0,3-0,15\right).2=0,3g\)

\(n_{Zn}=\dfrac{1,3}{65}=0,02\left(mol\right)\\
pthhZn+2HCl\rightarrow ZnCl_2+H_2\)
0,02 0,02 0,02
\(m_{ZnCl_2}=136.0,02=2,72\left(g\right)\\
V_{H_2}=0,02.22,4=0,448\left(l\right)\)

nO2 = 2,24/22,4 = 0,1 (mol)
PTHH: 2R + O2 -> (t°) 2RO
nRO = 0,1 . 2 = 0,2 (mol)
M(RO) = 16,2/0,2 = 81 (g/mol)
<=> R + 16 = 81
<=> R = 65
<=> R là Zn
\(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{2,24}{22,4}=0,1mol\)
\(m_{O_2}=n_{O_2}.M_{O_2}=0,1.32=3,2g\)
Vì R hóa trị II nên PTHH là:
\(2R+O_2\rightarrow\left(t^o\right)2RO\)
2 1 2 ( mol )
0,2 0,1
Áp dụng định luật bảo toàn khối lượng, ta có:
\(m_R=16,2-3,2=13g\)
\(M_R=\dfrac{m_R}{n_R}=\dfrac{13}{0,2}=65\) g/mol
\(\Rightarrow R\) là kẽm (Zn)
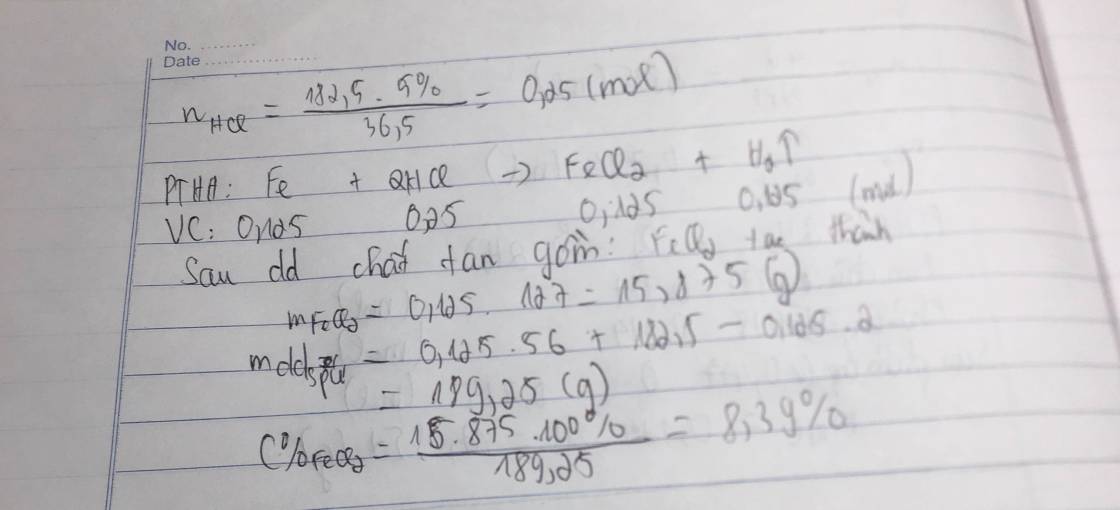
Fe+2HCl->Fecl2+H2
0,15---0,3-----------0,15
n Fe=0,15 mol
=>VH2=0,15.22,4=3,36l
2H2+O2-to>2H2O
0,15---------------0,15
n O2=0,2 mol
=>O2 dư
=>m H2O=0,15.18=2,7g