Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\\ n_{Al}=\dfrac{m}{M}=\dfrac{10,8}{27}=0,4\left(mol\right)\\ Theo.PTHH:n_{Al_2O_3}=\dfrac{1}{2}.n_{Al}=\dfrac{1}{2}.0,4=0,2\left(mol\right)\\ m_{Al_2O_3}=n.M=0,2.102=20,4\left(g\right)\)
\(b,n_{O_2}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ Lập.tỉ.lệ:\dfrac{n_{Al}}{4}>\dfrac{n_{O_2}}{3}\Rightarrow Al.dư\\ Theo.PTHH:n_{Al\left(pư\right)}=\dfrac{4}{3}.n_{O_2}=\dfrac{4}{3}.0,2\left(mol\right)\\ n_{Al\left(dư\right)}=n_{Al\left(bđ\right)}-n_{Al\left(pư\right)}=0,4-0,2=0,2\left(mol\right)\\ Theo.PTHH:n_{Al_2O_3}=\dfrac{1}{2}.n_{Al}=\dfrac{1}{2}.0,2=0,1\left(mol\right)\\ m_{Al_2O_3}=n.M=0,1=102=10,2\left(g\right)\)

PTHH:
4H2+Fe3O4----->3Fe+4H2O
nH2=V/22,4=6,72/22,4=0,3mol
Theo PTHH:4molH2--->3molFe 0,3molH2->0,3.3/4=0,225molFe
mFe=nFe.M=0,225.56=12,6g
nO= nH2O= nH2= 0,3(mol)
m=m(oxit) - mO= 24- 0,3.16= 19,2(g)

a)\(n_{Fe}=\dfrac{16,8}{56}=0,3mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,3 0,3 0,3
\(V_{H_2}=0,3\cdot22,4=6,67l\)
\(m_{FeCl_3}=0,3\cdot127=38,1g\)
b)\(n_{Fe_2O_3}=\dfrac{18}{160}=0,1125mol\)
\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
0,1125 0,3 0 0
0,1 0,3 0,2 0,3
0,0125 0 0,2 0,3
\(m_{Fe}=0,2\cdot56=11,2g\)

\(n_{Fe}=\dfrac{12.6}{56}=0.225\left(mol\right)\)
\(n_{O_2}=\dfrac{4.2}{22.4}=0.1875\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{^{^{t^0}}}Fe_3O_4\)
\(3.........2\)
\(0.225......0.1875\)
Lập tỉ lệ : \(\dfrac{0.225}{3}< \dfrac{0.1875}{2}\Rightarrow O_2dư\)
\(m_{O_2\left(dư\right)}=\left(0.1875-0.225\cdot\dfrac{2}{3}\right)\cdot32=1.2\left(g\right)\)
\(m_{Fe_3O_4}=\dfrac{0.225}{3}\cdot232=17.4\left(g\right)\)

`FeO + H_2` $\xrightarrow[]{t^o}$ `Fe + H_2 O`
`a) n_[H_2] = [ 3,36 ] / [ 22,4 ] = 0,15 (mol)`
`n_[FeO] = [ 14,2 ] / 72 = 71 / 360`
Ta có: `[ 0,15 ] / 1 < [ 71 / 360 ] / 1`
`=> FeO` dư
Theo `PTHH` có: `n_[FeO_\text{(p/ứ)}] = n_[H_2] = 0,15 (mol)`
`=> n_[FeO_\text{(dư)}] = 71 / 360 - 0,15 = 17 / 360 (mol)`
_______________________________________________
`b)` Theo `PTHH` có: `n_[Fe] = n_[H_2] = 0,15 (mol)`
`=> m_[Fe] = 0,15 . 56 = 8,4 (g)`

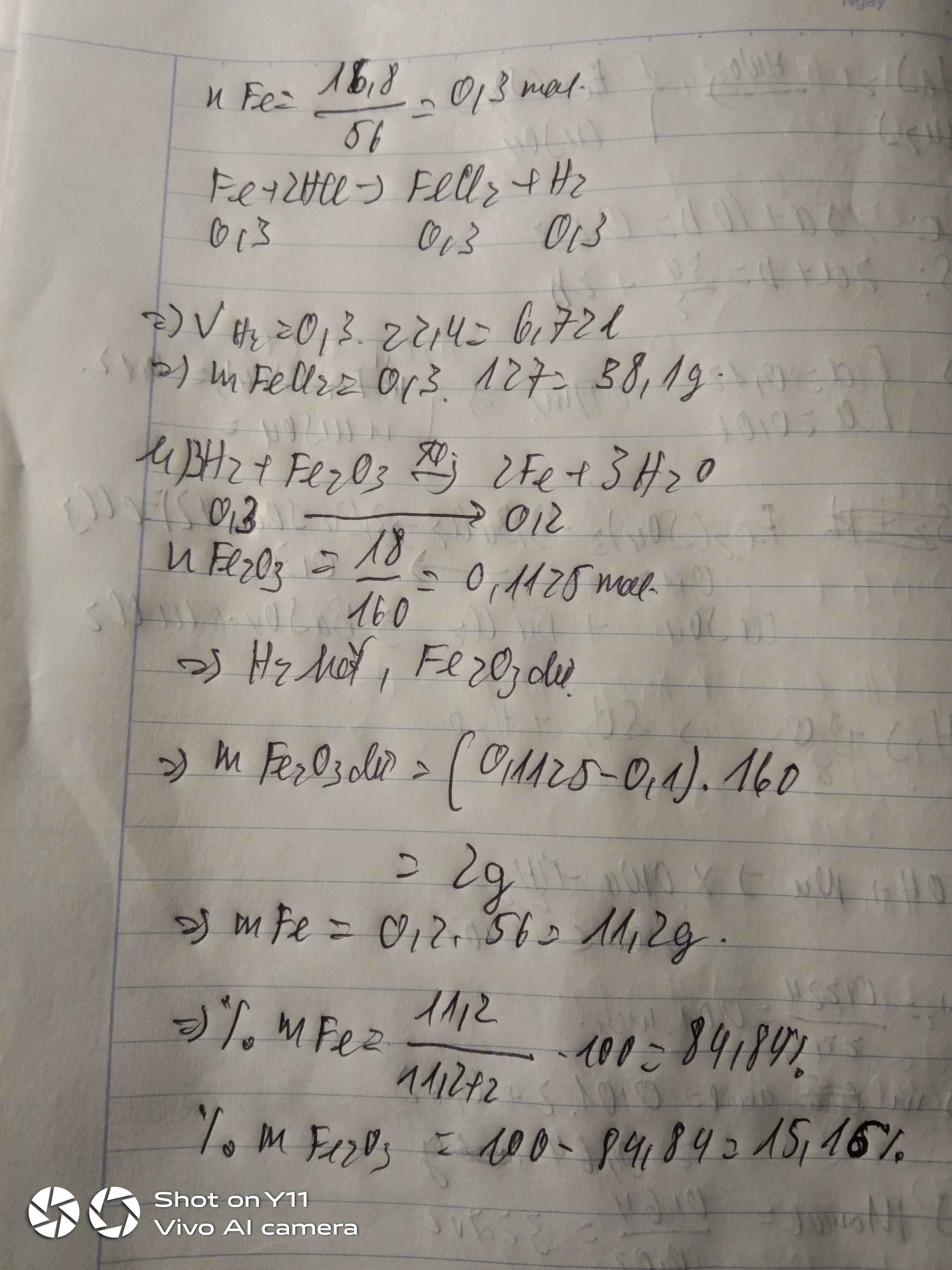
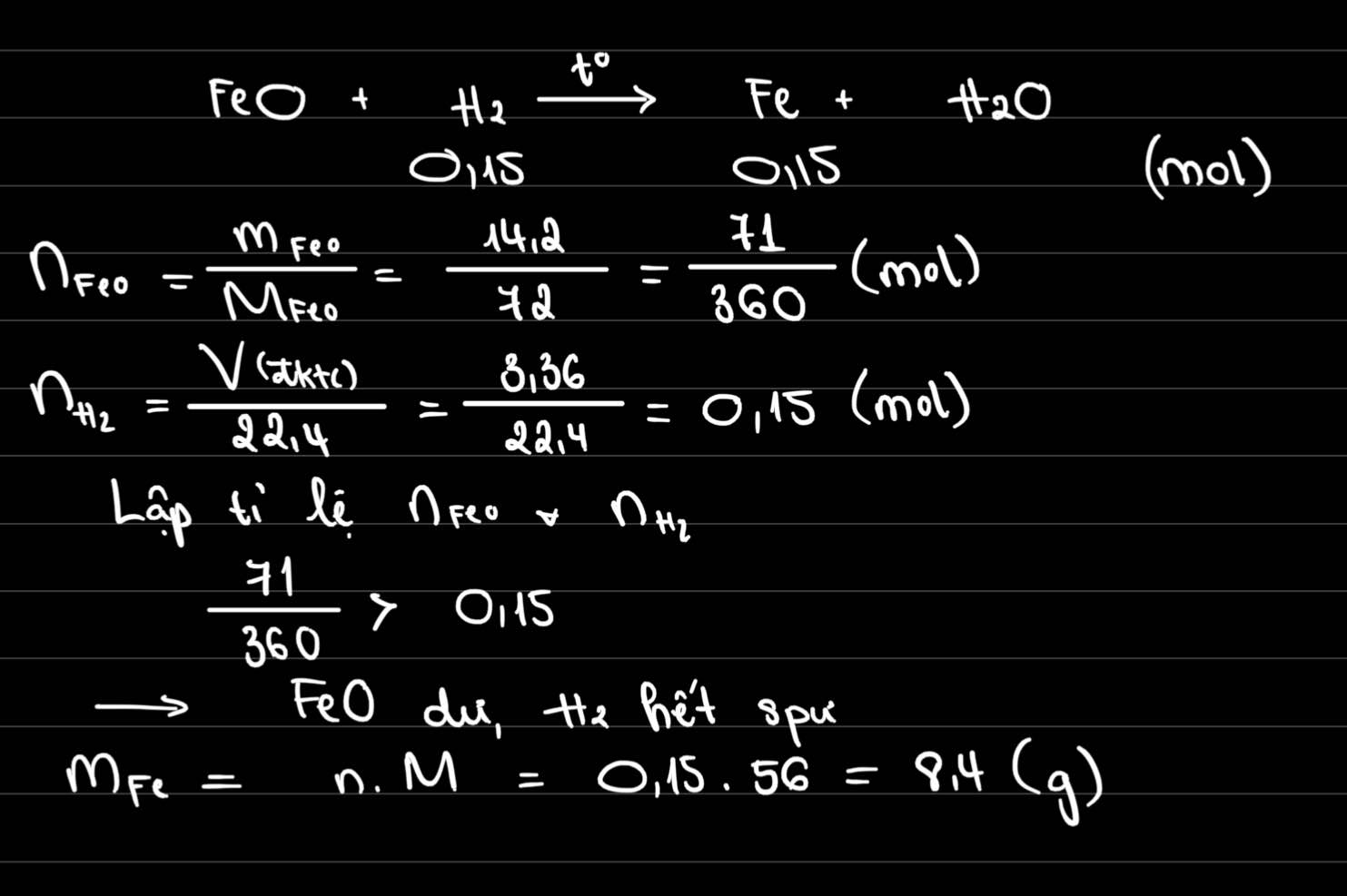
\(a,PTHH:Fe_3O_4+4H_2\xrightarrow{t^o}3Fe+4H_2O\\ n_{H_2}=\dfrac{6,72}{22,4}=0,3(mol);n_{Fe_3O_4}=\dfrac{46,4}{232}=0,2(mol)\)
Vì \(\dfrac{n_{H_2}}{4}<\dfrac{n_{Fe_3O_4}}{1}\) nên \(Fe_3O_4\) dư
\(n_{Fe_3O_4(dư)}=0,2-\dfrac{0,3}{4}=0,125(mol)\\ \Rightarrow m_{Fe_3O_4(dư)}=0,125.232=29(g)\\ b,n_{Fe}=\dfrac{3}{4}n_{H_2}=0,225(mol)\\ \Rightarrow m_{Fe}=0,225.56=12,6(g)\)