Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(m_{HCl}=\dfrac{200\cdot14,6\%}{100\%}=29,2g\Rightarrow n_{HCl}=0,8mol\)
a)\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,8 0 0
0,1 0,2 0,1 0,1
0 0,6 0,1 0,1
b)Chất HCl dư và dư \(m=0,6\cdot36,5=21,9g\)
c)\(V_{H_2}=0,1\cdot22,4=2,24l\)
d)\(m_{H_2}=0,1\cdot2=0,2g\)
\(m_{ZnCl_2}=0,1\cdot136=13,6g\)
\(m_{ddZnCl_2}=6,5+200-0,2=206,3g\)
\(C\%=\dfrac{13,6}{206,3}\cdot100\%=6,59\%\)
a, ta có pt sau : Zn + 2HCl >ZnCl2 + H2 (1)
b, nHCl=\(\dfrac{200\times14,6}{100}=29,2\left(g\right)\)\(\Rightarrow n_{HCl}=\dfrac{29,2}{36,5}=0,8\left(mol\right)\)
Ta có : nZn=\(\dfrac{6,5}{65}=0,1\left(mol\right)\)
Ta có tỉ lệ số mol là : \(\dfrac{n_{Zn}}{1}< \dfrac{n_{HCl}}{2}\left(\dfrac{0,1}{1}< \dfrac{0,8}{2}\right)\)
\(\Rightarrow\) HCl dư , Zn pứ hết
Theo pt : nHClpứ = 2.nZn=2.0,1=0,2(mol)
\(\Rightarrow\)nHCl dư = nHCl bđ - nHCl pứ = 0,8 - 0,2 = 0,6 (mol)
\(\Rightarrow\)mHCl dư=0,6.36,6=21,9 (g)
c,theo pt :nH2=nZn=0,1(mol)
\(\Rightarrow\)VH2=0,1.22,4=2,24(l)
d,Các chất có trong dung dịch sau pứ là: ZnCl2 , HCl dư
mk chịu câu này ![]()

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
\(m_{HCl}=100.14,6\%=14,6\left(g\right)\Rightarrow n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{0,4}{2}\), ta được HCl dư.
Theo PT: \(n_{H_2}=n_{Zn}=0,1\left(mol\right)\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, Theo PT: \(\left\{{}\begin{matrix}n_{HCl\left(pư\right)}=2n_{Zn}=0,2\left(mol\right)\\n_{ZnCl_2}=n_{Zn}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{HCl\left(dư\right)}=0,4-0,2=0,2\left(mol\right)\)
Ta có: m dd sau pư = 6,5 + 100 - 0,1.2 = 106,3 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,1.136}{106,3}.100\%\approx12,79\%\\C\%_{HCl\left(dư\right)}=\dfrac{0,2.36,5}{106,3}.100\%\approx6,87\%\end{matrix}\right.\)

a. \(Mg+2HCl\rightarrow MgCl_2+H_2\)
b. \(n_{Mg}=\dfrac{2.4}{24}=0.1mol\)
\(mct_{HCl}=\dfrac{500\times36.5}{100}=182.5g\Rightarrow n_{HCl}=\dfrac{182.5}{36.5}=5mol\)
Ta có: \(\dfrac{0.1}{1}< \dfrac{5}{2}\Rightarrow\) HCl dư
nHCl phản ứng = 0.2 mol => nHCl dư = 5 - 0.2 = 4.8 mol
mHCl dư = \(4.8\times36.5=175.2g\)
c. \(V_{H_2}=0.1\times22.4=2.24l\)
d. mdd sau phản ứng = \(2.4+500-0.1\times2=502.2g\)
\(C\%_{MgCl_2}=\dfrac{0.1\times95\times100}{502.2}=1.89\%\)

\(n_{Zn}=\dfrac{13}{65}=0.2\left(mol\right)\)
\(n_{HCl}=\dfrac{182.5\cdot10}{100\cdot36.5}=0.5\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.2......0.4..........0.2........0.2\)
\(n_{HCl\left(dư\right)}=0.5-0.4=0.1\left(mol\right)\)
\(m_{HCl\left(dư\right)}=0.1\cdot36.5=3.65\left(g\right)\)
\(V_{H_2}=0.2\cdot22.4=4.48\left(l\right)\)
\(m_{\text{dung dịch sau phản ứng}}=13+182.5-0.2\cdot2=195.1\left(g\right)\)
\(C\%_{HCl\left(dư\right)}=\dfrac{3.65}{195.1}\cdot100\%=1.87\%\)
\(C\%_{ZnCl_2}=\dfrac{0.2\cdot136}{195.1}\cdot100\%=13.94\%\)

\(a)n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\\ 2Al+6HCl\xrightarrow[]{}2AlCl_3+3H_2\\ n_{H_2}=\dfrac{3}{2}n_{Al}=\dfrac{3}{2}\cdot0,4=0,6\left(mol\right)\\ V_{H_2}=0,6.22,4=13,44\left(l\right)\\ b)n_{HCl}=3n_{Al}=3.0,4=1,2\left(mol\right)\\ m_{HCl}=1,2.36,5=43,8\left(g\right)\\ m_{dd_{HCl}}=\dfrac{43,8}{10,95\%}\cdot100\%=400\left(g\right)\\ c)n_{AlCl_3}=n_{Al}=0,4mol\\ m_{AlCl_3}=0,4.133,5=53,4\left(g\right)\\ m_{H_2}=0,6.2=1,2\left(g\right)\\ m_{dd_{AlCl_3}}=10,8+400-1,2=409,6\left(g\right)\\ C_{\%AlCl_3}=\dfrac{53,4}{409,6}\cdot100\%\approx13\%\)

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

Sửa đề: 8,4 gam Fe
\(a,n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ n_{HCl}=\dfrac{14,6.175}{36,5.100}=0,7\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
ban đầu 0,15 0,7
phản ứng 0,15 0,3
sau pư 0 0,4 0,15 0,15
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
\(b,m_{dd}=8,4+175-0,15.2=183,1\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}C\%_{FeCl_2}=\dfrac{0,15.127}{183,1}.100\%=10,4\%\\C\%_{HCl\left(dư\right)}=\dfrac{0,4.36,5}{183,1}.100\%=7,97\%\end{matrix}\right.\)

a)
\(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(m_{HCl}=\dfrac{175.14,6}{100}=25,55\left(g\right)\\ \rightarrow n_{HCl}=\dfrac{25,55}{35,5}=0,7\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
bđ 0,3 0,7
pư 0,3 0,6
spư 0 0,1 0,3 0,3
=> VH2 = 0,3.22,4 = 6,72 (l)
b)
mdd = 16,8 + 175 - 0,3.2 = 191,2 (g)
=> \(\left\{{}\begin{matrix}C\%_{FeCl_2}=\dfrac{0,3.127}{191,2}.100\%=19,93\%\\C\%_{HCl\left(dư\right)}=\dfrac{0,1.36,5}{191,2}.100\%=1,91\%\end{matrix}\right.\)
\(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\\ n_{HCl}=\dfrac{\dfrac{175.14,6}{100}}{36,5}=0,7\left(mol\right)\\ pthh:Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
\(LTL:\dfrac{0,3}{1}< \dfrac{0,7}{2}\)
\(n_{H_2}=n_{Fe}=0,3\left(mol\right)\\
V_{H_2}=0,3.22,4=6,72\left(l\right)\\
m_{\text{dd}}=16,8+175-\left(0,3.2\right)=191,2\left(g\right)\\
n_{FeCl_2}=n_{Fe}=0,3\left(mol\right)\\
C\%_{FeCl_2}=\dfrac{0,3.127}{191,2}.100\%=19,92\%\)
=> HCl dư
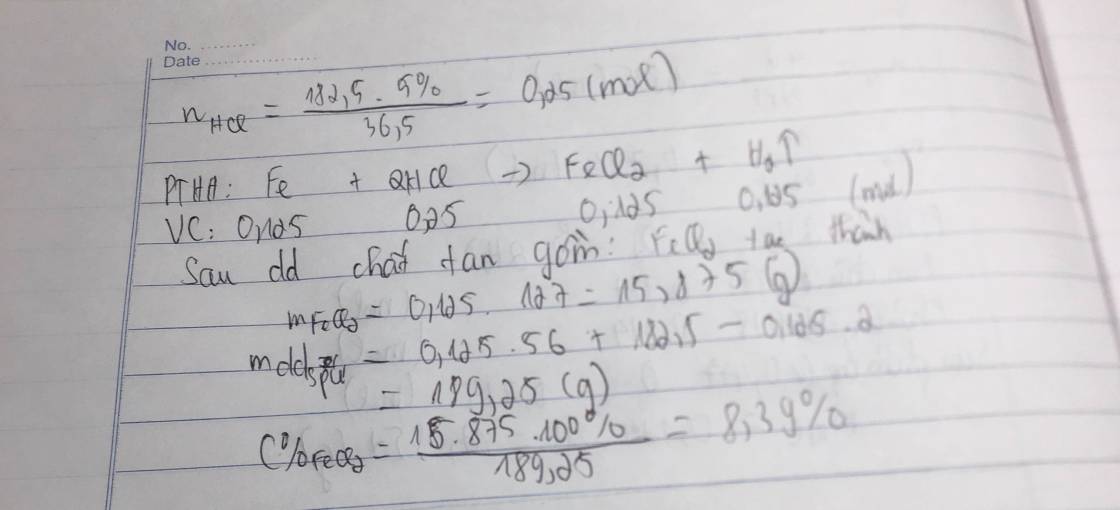
\(n_{Zn}=\dfrac{6.5}{65}=0.1\left(mol\right)\)
\(n_{HCl}=\dfrac{100\cdot14.6\%}{36.5}=0.4\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(1........2\)
\(0.1......0.4\)
\(LTL:\dfrac{0.1}{1}< \dfrac{0.4}{2}\Rightarrow HCldư\)
\(V_{H_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(m_{\text{dung dịch sau phản ứng}}=6.5+100-0.1\cdot2=106.3\left(g\right)\)
\(C\%ZnCl_2=\dfrac{0.1\cdot136}{106.3}\cdot100\%=12.79\%\)
\(C\%HCl\left(dư\right)=\dfrac{\left(0.4-0.2\right)\cdot36.5}{106.3}\cdot100\%=6.87\%\%\)
cảm ơn bạn nhiều .