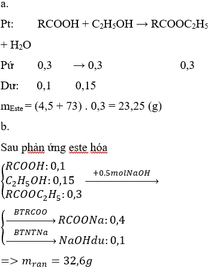
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
\(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
0,1 0,2
a. \(V_{CH_3COOH}=\dfrac{0,2}{1}=0,2\left(l\right)\)
b. \(CH_3COOH+C_2H_5OH⇌\left(H_2SO_{4đ},t^o\right)CH_3COOC_2H_5+H_2O\)
0,2 0,2
Với H% = 80
\(m_{CH_3COOC_2H_5}=\dfrac{0,2.88.80}{100}=14,08\left(g\right)\)

a.\(n_{\left(CH_3COO\right)_2Mg}=\dfrac{14,2}{142}=0,1mol\)
\(2CH_3COOH+Mg\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
0,2 0,1 0,1 ( mol )
\(C_{M_{CH_3COOH}}=\dfrac{0,2}{0,25}=0,8M\)
\(V_{H_2}=0,1.22,4=2,24l\)
b.\(NaOH+CH_3COOH\rightarrow CH_3COONa+H_2O\)
0,2 0,2 ( mol )
\(V_{NaOH}=\dfrac{0,2}{0,5}=0,4l\)
\(n_{\left(CH_3COO\right)_2Mg}=\dfrac{14,2}{142}=0,1\left(mol\right)\)
PTHH: 2CH3COOH + Mg ---> (CH3COO)2Mg + H2
0,2<---------------------------0,1---------->0,1
=> \(\left\{{}\begin{matrix}C_{M\left(CH_3COOH\right)}=\dfrac{0,2}{0,25}=0,8M\\V_{H_2}=0,1.22,4=2,4\left(l\right)\end{matrix}\right.\)
PTHH: CH3COOH + NaOH ---> CH3COONa + H2O
0,2------------->0,2
=> \(V_{ddNaOH}=\dfrac{0,2}{0,5}=0,4\left(l\right)\)

a, Ta có: \(n_{CH_3COOH}=\dfrac{9,6}{60}=0,16\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
Theo PT: \(n_{\left(CH_3COO\right)_2Mg}=\dfrac{1}{2}n_{CH_3COOH}=0,08\left(mol\right)\)
\(\Rightarrow m_{\left(CH_3COO\right)_2Mg}=0,08.142=11,36\left(g\right)\)
b, PT: \(CH_3COOH+C_2H_5OH\underrightarrow{t^o,xt}CH_3COOC_2H_5+H_2O\)
Theo PT: \(n_{CH_3COOC_2H_5\left(LT\right)}=n_{CH_3COOH}=0,16\left(mol\right)\)
\(\Rightarrow m_{CH_3COOC_2H_5\left(LT\right)}=0,16.88=14,08\left(g\right)\)
Mà: thực tế thu được 10,56 (g)
\(\Rightarrow H\%=\dfrac{10,56}{14,08}.100\%=75\%\)

a) \(n_{\left(CH_3COO\right)_2Mg}=\dfrac{1,42}{142}=0,01\left(mol\right)\)
PTHH: Mg + 2CH3COOH --> (CH3COO)2Mg + H2
0,02<-----------0,01-------->0,01
=> VH2 = 0,01.22,4 = 0,224 (l)
\(C_{M\left(CH_3COOH\right)}=\dfrac{0,02}{0,2}=0,1M\)
b)
PTHH: CH3COOH + NaOH --> CH3COONa + H2O
0,02------>0,02
=> \(V_{dd.NaOH}=\dfrac{0,02}{0,2}=0,1\left(l\right)=100\left(ml\right)\)

\(n_{\left(CH_3COO\right)_2Mg}=\dfrac{2,84}{142}=0,02\left(mol\right)\)
PTHH :
\(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\uparrow\)
0,04 0,02 0,02
\(a,C_M=\dfrac{n}{V}=\dfrac{0,04}{0,1}=0,4M\)
\(b,V_{H_2}=0,02.22,4=0,448\left(l\right)\)
\(c,PTHH:\)
\(CH_3COOH+C_2H_5OH\underrightarrow{t^o,H_2SO_{4\left(đ\right)}}CH_3COOC_2H_5+H_2O\)
0,04 0,04
\(m_{este}=0,04.90\%.88=3,168\left(g\right)\)

2CH3COOH + Zn -- > (CH3COOH)2Zn + H2
nH2 = 2,24 / 22,4 = 0,1 (mol)
=> nCH3COOH = 0,2 (mol)
mZn = 0,1. 65 = 6,5 (g)
mH2 = 0,1.2 = 0,2 (g)
mdd = 300 + 6,5 - 0,2 = 306,3 (g)
mCH3COOH = 0,2 . 60 = 12 (g)
=> C%CH3COOH = ( 12.100 ) / 306,3 = 4%
m(CH3COO)2Zn = 0,1 . 183 = 18,3 (g)
=> (18,3.100) / 306,3 = 6%

\(a,n_{\left(CH_3COO\right)_2Mg}=\dfrac{7,1}{142}=0,05\left(mol\right)\)
PTHH: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\uparrow\)
0,1<----------------0,05-------------->0,05
\(\rightarrow V_{H_2}=0,05.22,4=1,12\left(l\right)\\ b,C\%_{CH_3COOH}=\dfrac{0,1.60}{100}.100\%=6\%\)
\(c,n_{C_2H_5OH}=\dfrac{6,9}{46}=0,15\left(mol\right)\)
PTHH: \(CH_3COOH+C_2H_5OH\xrightarrow[H_2SO_{4\left(đặc\right)}]{t^o}CH_3COOC_2H_5+H_2O\)
bđ 0,1 0,15
pư 0,1 0,1
spư 0 0,05 0,1
\(\rightarrow m_{este}=0,1.80\%.88=7,04\left(g\right)\)

nKOH = 0,5.0,3 = 0,15 mol
CH3COOH + KOH → CH3COOK + H2O
0,15 0,15 0,15 mol
a) CM CH3COOH = 0,15/0,2 =0,75M
b) Thể tích của dung dịch thu được sau phản ứng: 500 ml
CM CH3COOK = 0,15/0,5 = 0,3M
c) Phản ứng lên men giấm
C2H5OH + O2 → CH3COOH + H2O
0,15 0,15
→ mC2H5OH = 0,15.46 = 6,9 gam
\(n_{KOH}=0,5\cdot0,3=0,15mol\)
\(CH_3COOH+KOH\rightarrow CH_3COOK+H_2O\)
0,15 0,15 0,15 0,15
a)\(C_{M_{CH_3COOH}}=\dfrac{0,15}{0,2}=0,75M\)
b)\(C_{M_{CH_3COOK}}=\dfrac{0,15}{0,2+0,3}=0,3M\)