Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1:
\(n_{HCl}=2.0,16=0,32\left(mol\right);n_{H_2}=\dfrac{3,584}{22,4}=0,16\left(mol\right)\)
PTHH: Mg + 2HCl → MgCl2 + H2
PTHH: Fe + 2HCl → FeCl2 + H2
\(m_{H_2}=0,16.2=0,32\left(g\right)\)
\(m_{HCl}=0,32.36,5=11,68\left(g\right)\)
Theo ĐLBTKL ta có: \(m_{MgCl_2+FeCl_2}=1,4+11,68-0,32=12,76\left(g\right)\)
Bài 12:
Theo ĐLBTKL, ta có:
\(m_{hhkl}+m_{O_2}=m_{hh.oxit}\\ \Leftrightarrow11,9+m_{O_2}=18,3\\ \Leftrightarrow m_{O_2}=18,3-11,9=6,4\left(g\right)\\ n_{O_2}=\dfrac{6,4}{32}=0,2\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\)

Gọi số mol Al, Fe là a, b (mol)
=> 27a + 56b = 11,1 (1)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
a----------------------->1,5a
Fe + 2HCl --> FeCl2 + H2
b------------------------>b
=> 1,5a + b = 0,3 (2)
(1)(2) => a = 0,1; b = 0,15
=> \(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{11,1}.100\%=24,32\%\\\%m_{Fe}=\dfrac{0,15.56}{11,1}.100\%=75,68\end{matrix}\right.\)

\(4.\)
\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.15.....0.3....................0.15\)
\(m_{Fe}=0.15\cdot56=8.4\left(g\right)\)
\(C_{M_{HCl}}=\dfrac{0.3}{0.5}=0.6\left(M\right)\)
\(5.\)
\(Đặt:n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(m_{hh}=56a+27b=8.3\left(g\right)\left(1\right)\)
\(n_{H_2}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(\Rightarrow a+1.5b=0.25\left(2\right)\)
\(\left(1\right),\left(2\right):a=b=0.1\)
\(\%Fe=\dfrac{5.6}{8.3}\cdot100\%=67.47\%\)
\(\%Al=32.53\%\)
bạn ơi cho mik hỏi: tại sao lại suy ra: a+1,5b=0,25 vậy ạ ? và cả bước tiếp theo nx ạ ?

Sửa: $V_{H_2}=7,168(l)$
$a\bigg)$
Đặt $n_{Mg}=x;n_{Fe}=y;n_{Al}=z$
$\to 24x+56y+27z=9,52(1)$
$n_{H_2}=\dfrac{7,168}{22,4}=0,32(mol)$
$n_{Cl_2}=\dfrac{8,064}{22,4}=0,36(mol)$
BTe: $x+y+1,5z=n_{H_2}=0,32(2)$
BTe: $x+1,5y+1,5z=n_{Cl_2}=0,36(3)$
Từ $(1)(2)(3)\to x=0,12(mol);y=0,08(mol);z=0,08(mol)$
$\to \begin{cases} \%m_{Mg}=\dfrac{0,12.24}{9,52}.100\%=30,25\%\\ \%m_{Fe}=\dfrac{0,08.56}{9,52}.100\%=47,06\%\\ \%m_{Al}=100-47,06-30,25=22,69\% \end{cases}$
$b\bigg)$
Bảo toàn H: $n_{HCl}=2n_{H_2}=0,64(mol)$
$\to C_{M_{HCl}}=\dfrac{0,64}{0,2}=3,2M$
$\to a=3,2$
$c\bigg)$
Dung dịch sau gồm $MgCl_2,FeCl_2,AlCl_3$
Bảo toàn $Mg,Al,Fe:n_{MgCl_2}=0,12(mol);n_{AlCl_3}=n_{FeCl_2}=0,08(mol)$
$\to C_{M_{MgCl_2}}=\dfrac{0,12}{0,2}=0,6M$
$\to C_{M_{AlCl_3}}=C_{M_{FeCl_2}}=\dfrac{0,08}{0,2}=0,4M$
$a\bigg)$
Đặt $n_{Mg}=x;n_{Fe}=y;n_{Al}=z$
$\to 24x+56y+27z=9,52(1)$
$n_{H_2}=\dfrac{14,336}{22,4}=0,64(mol)$
$n_{Cl_2}=\dfrac{8,064}{22,4}=0,36(mol)$
BTe: $x+y+1,5z=n_{H_2}=0,64(2)$
BTe: $x+1,5y+1,5z=n_{Cl_2}=0,36(3)$
Từ $(1)(2)(3)\to$ nghiệm âm, xem lại đề

\(n_{Cl_2}=\dfrac{13.44}{22.4}=0.6\left(mol\right)\)
\(n_{H_2}=\dfrac{11.2}{22.4}=0.5\left(mol\right)\)
\(n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(n_{H_2}=a+1.5b=0.5\left(mol\right)\)
\(n_{Cl_2}=1.5a+1.5b=0.6\left(mol\right)\)
\(\Rightarrow a=b=0.2\)
\(m_X=0.2\cdot\left(56+27\right)=16.6\left(g\right)\)

Phản ứng xảy ra:
\(2Al+6HCl\rightarrow2Alcl_3+3H_2\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
Ta có:
\(n_{H_2}=\frac{3,36}{22,4}=0,15mol\)
\(\rightarrow n_{Al}=\frac{2}{3}n_{H_2}=0,1mol\)
\(\rightarrow m_{Al}=0,1.27=2,7gam\rightarrow m_{Al_2O_2}=10,2gam\)
\(\rightarrow\%m_{Al}=\frac{2,7}{12,9}=20,93\%\rightarrow\%m_{Al_2O_2}=79,07\%\)
\(n_{Al_2O_3}=\frac{10,2}{27.2+16.3}=0,1mol\)
\(\rightarrow n_{HCl}=3n_{Al}+6n_{Al_2O_3}=0,1.3+0,1.6=0,9mol\)
\(\rightarrow m_{HCl}=0,9.36,5=32,85gam\)
\(\rightarrow m_{ddHCl}=\frac{32,85}{3,65\%}=900gam\)

Gọi số mol Mg, Fe, Al là a, b, c
=> 24a + 56b + 27c = 23,8
PTHH: Mg + 2HCl --> MgCl2 + H2
a------------------------->a
Fe + 2HCl --> FeCl2 + H2
b------------------------->b
2Al + 6HCl --> 2AlCl3 + 3H2
c------------------------->1,5c
=> a + b + 1,5c = \(\dfrac{17,92}{22,4}=0,8\left(mol\right)\)
PTHH: Mg + Cl2 --to--> MgCl2
a-->a
2Fe + 3Cl2 --to--> 2FeCl3
b--->1,5b
2Al + 3Cl2 --to--> 2AlCl3
c--->1,5c
=> \(a+1,5b+1,5c=\dfrac{20,16}{22,4}=0,9\left(mol\right)\)
=> a = 0,3; b = 0,2; c = 0,2
=> \(\left\{{}\begin{matrix}m_{Mg}=0,3.24=7,2\left(g\right)\\m_{Fe}=0,2.56=11,2\left(g\right)\\m_{Al}=0,2.27=5,4\left(g\right)\end{matrix}\right.\)
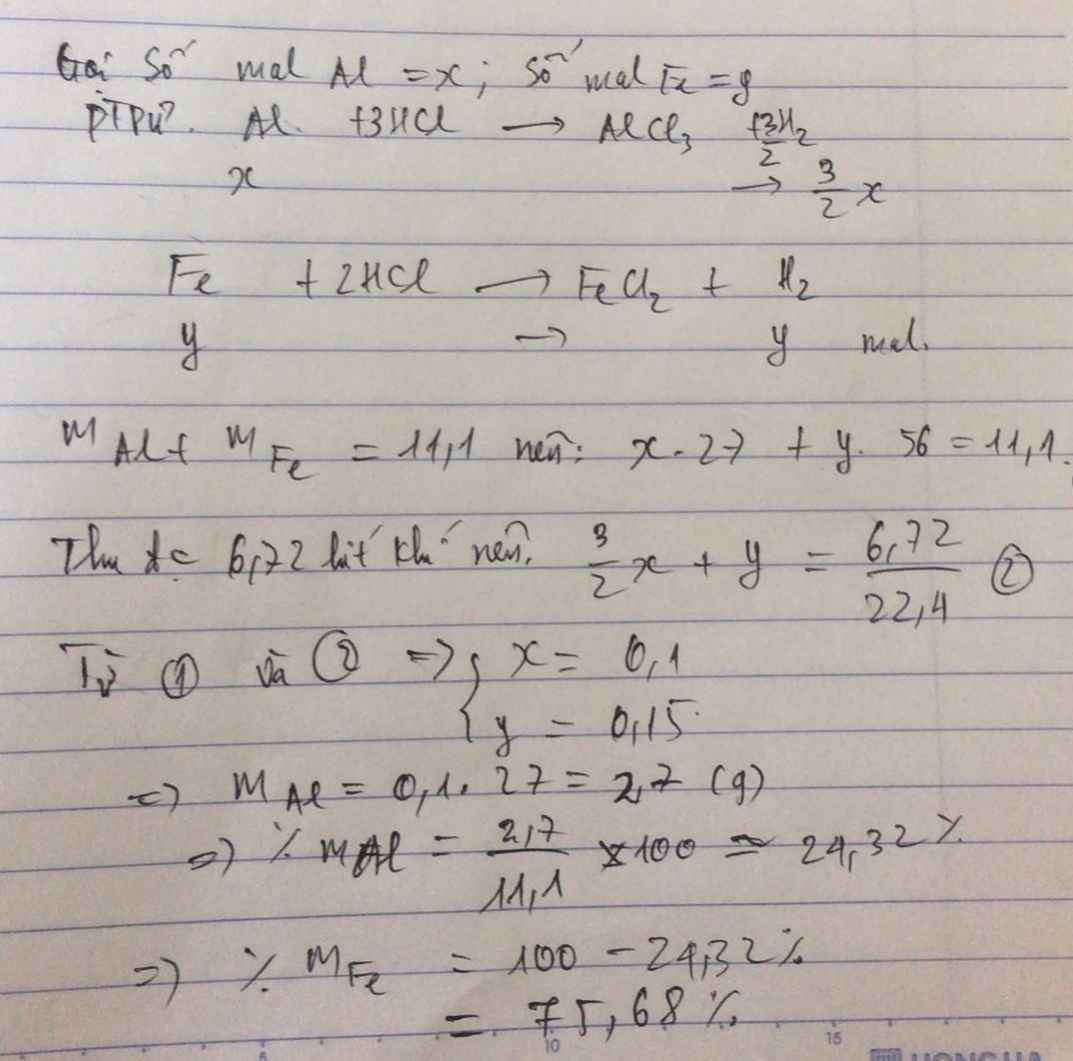
\(n_{HCl}=0.5\cdot1=0.5\left(mol\right)\)
\(n_{H_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(0.15.....0.3........................0.15\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(0.1.........0.5-0.3\)
\(m_A=0.15\cdot24+0.1\cdot80=11.6\left(g\right)\)